Chemistry:Nitrosyl bromide
| |||
|
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| NOBr | |||
| Molar mass | 109.910 g/mol | ||
| Appearance | Red gas | ||
| Boiling point | 14.5 °C (58.1 °F; 287.6 K) | ||
Refractive index (nD)
|
1.524 | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
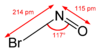
Nitrosyl bromide is the chemical compound with the chemical formula NOBr. It is a red gas with a condensing point just below room temperature.[1] It reacts with water.[1]
Nitrosyl bromide can be formed by the reversible reaction of nitric oxide with bromine.[2] This reaction is of interest as it is one of very few third-order homogeneous gas reactions. NOBr is prone to photodissociation at standard pressure and temperature.
- 2 NO + Br2 ⇌ 2 NOBr
Another way to make it is by way of nitric oxide reacting with potassium bromide.[1]
- 2NO2 + KBr → BrNO + KNO3
Experiment
The bond breaking of the chemical can be done with photolysis using a light to separate the molecules that are present. Another to separate nitrosyl bromide into NO and Br or Br2 is by having excess of NO which then the experiment will follow first order kinetics. This reverse rate constant was calculated to be kr = 2.29 ± 0.33 x 10-21 cm3 /molecules
With excess Br2 plus NO the reaction follows third order kinetics.
Br2 + 2NO ↔ 2BrNO
There was a rate constant found of kf = 1.56 ± 0.20 x 10-38 cm6 /molecule2 - s at 293 ± 1 K[3]
More Information
The bone between N-O is an excited state while the bond between N-Br is repulsive. Since the bond between N-Br is repulsive that is why the bromine will dissociate from the chemical versus oxygen.
Nitrosyl bromide can be formed in the atmosphere. Since Br is a halogen that is linked to chemical reactions that late deplete the ozone. This chemical is important to study because of the ozone depletion caused by bromine in the atmosphere. If Bromide is instead nitrosyl bromide or bromine it does not aid in the deplete of the ozone layer.
Third order reaction
The third order reaction is the best reaction to show the formation of nitrosyl bromide. The third order reaction is rare to see with bond breaking reactions between stable molecules but there have been no experiments to prove that this experiment does not have any intermediate steps, but it is suspected that there is two steps. [3]
Safety Precautions
Making and photodissociation can lead to health hazards. Working in a station where the gas and liquid will not get into contact with your skin and eyes is important. Bromine is volatizes rapidly to form a red vapor which can be irritating to both the eyes and throat. The liquid form of bromine can cause sores on the skin as well. While nitric oxide is a gas and is very toxic to come into contact with along with nitric dioxide which can also be rapidly formed. Is a poisonous brown vapor that is also a component of smog.[3] Making sure to work in a fume hood along with wearing eye protection along with gloves, long sleeves, pants, and close toed shoes is the best way to work in forming this chemical.
Physical Appearance
Nitric Oxide is a colorless gas at room temperature Bromine is a dark red liquid at room temperature, but volatizes rapidly to form a red vapor that has a strong odor. Nitrosyl bromide is a red gas at room temperate. Note do not breath or waft any of these chemicals because they are dangerous to your health.
History
Some of the previous experiments that determined reaction rates were also done by Histatsune and Zafonte, Hippler, and Godfrey. Histatsune and Zafonte determined the forward and reverse reaction rate constants. Hippler studied the recombination of Br atoms after photoylsis of less than 0.3 torr of Br2 at room temperate in the range of 1 - 100 atm. He also studied the 2 recombination of Br and NO in the presence of helium. Godfrey examined the kinetics of BrNO formation and destruction using time resolved photolysis techniques. He also included the effects of the loss of Br2 to internal surfaces of the cell in calculations of the reaction rate constants. The reaction rates were determined to be between the range of 1.32 ± 0.14 to 1.68 ± 0.11 x 10-38 cm6 /molecule2 -s for kf and from 2.09 ± 0.55 to 3.71 x 10-21 cm3 /molecule-s for kr.
Further Research
Further research could be done to fine the rate of decomposition.
References[4][5]
- ↑ 1.0 1.1 1.2 Ratcliffe, Charles T.; Shreeve, Jean'ne M.; Wynne, Kenneth J. (January 1968). "Nitrosyl Halides". Inorganic Syntheses. 11. pp. 194–200. doi:10.1002/9780470132425.ch39. ISBN 9780470131701.
- ↑ Esposti, C.D.; Tamassia, F.; Cazzoli, G.; Kisiel, Z. (April 1995). "Millimeter-Wave Spectrum of Nitrosyl Bromide in the Low-Lying Excited States: Equilibrium Structure and Cubic Force Field". Journal of Molecular Spectroscopy 170 (2): 582–600. doi:10.1006/jmsp.1995.1093. Bibcode: 1995JMoSp.170..582E.
- ↑ 3.0 3.1 3.2 Y. Lian et al., Theoretical study on the structure and dissociation mechanism of electronic excited states of nitrosyl bromide molecules. The Journal of Physical Chemistry A. 126, 2936–2941 (2022).
- ↑ Mahoney, Lori A., "The Kinetics Following Photolysis of Nitrosyl Bromide" (2004). Theses and Dissertations. 3719. https://scholar.afit.edu/etd/3719
- ↑ 1. C. M. Blair, P. D. Brass, D. M. Yost, The equilibrium between nitric oxide, bromine and nitrosyl bromide. Journal of the American Chemical Society. 56, 1916–1918 (1934).
External links
 |
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named:0