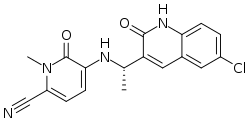
Chemistry:Olutasidenib
 | |
| Clinical data | |
|---|---|
| Trade names | Rezlidhia |
| Other names | FT-2102 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C18H15ClN4O2 |
| Molar mass | 354.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Olutasidenib, sold under the brand name Rezlidhia, is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation.[1][2] Olutasidenib is an isocitrate dehydrogenase-1 (IDH1) inhibitor.[1] It is taken by mouth.[1]
The most common adverse reactions include nausea, fatigue/malaise, arthralgia, constipation, leukocytosis, dyspnea, fever, rash, mucositis, diarrhea, and transaminitis.[3]
Olutasidenib was approved for medical use in the United States in December 2022,[1][2][3][4][5][6] based on the phase 1 results of a phase 1/2 trial.[7]
Medical uses
Olutasidenib is indicated for the treatment of adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.[1][2][3]
Society and culture
Names
Olutasidenib is the international nonproprietary name.[8]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Rezlidhia- olutasidenib capsule". DailyMed. U.S. National Library of Medicine. 13 December 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4a0c7c8b-b95f-455d-9600-b7351e4397fe.
- ↑ 2.0 2.1 2.2 2.3 "REZLIDHIA (olutasidenib) capsules". Approval Letter. U.S. Food and Drug Administration. December 2022. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215814Orig1s000ltr.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 3.2 "FDA approves olutasidenib for relapsed or refractory acute myeloid leukemia with a susceptible IDH1 mutation". 1 December 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olutasidenib-relapsed-or-refractory-acute-myeloid-leukemia-susceptible-idh1-mutation.
- ↑ "Rigel Announces U.S. FDA Approval of Rezlidhia (olutasidenib) for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia with a Susceptible IDH1 Mutation". Rigel Pharmaceuticals, Inc. (Press release). 1 December 2022. Retrieved 2 December 2022.
- ↑ "Rigel Announces U.S. FDA Approval of Rezlidhia (olutasidenib) for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia with a Susceptible IDH1 Mutation" (Press release). Rigel Pharmaceuticals. 1 December 2022. Retrieved 2 December 2022 – via PR Newswire.
- ↑ "Olutasidenib: First Approval". Drugs 83 (4): 341–346. March 2023. doi:10.1007/s40265-023-01844-1. PMID 36848032. https://figshare.com/articles/online_resource/Olutasidenib_First_Approval/22114562.
- ↑ Watts, Justin M; Baer, Maria R; Yang, Jay; Prebet, Thomas; Lee, Sangmin; Schiller, Gary J; Dinner, Shira N; Pigneux, Arnaud et al. (January 2023). "Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: phase 1 results of a phase 1/2 trial". The Lancet Haematology 10 (1): e46–e58. doi:10.1016/s2352-3026(22)00292-7. ISSN 2352-3026. PMID 36370742. http://dx.doi.org/10.1016/s2352-3026(22)00292-7.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 82". WHO Drug Information 33 (3). 2019.
Further reading
- "Isocitrate dehydrogenase inhibitors in acute myeloid leukemia". Biomarker Research 7: 22. 2019. doi:10.1186/s40364-019-0173-z. PMID 31660152.
- "Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: phase 1 results of a phase 1/2 trial". The Lancet Haematology 10 (1): e46–e58. November 2022. doi:10.1016/S2352-3026(22)00292-7. PMID 36370742.
External links
- "Olutasidenib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/olutasidenib.
- Clinical trial number NCT02719574 for "Open-label Study of FT-2102 With or Without Azacitidine or Cytarabine in Patients With AML or MDS With an IDH1 Mutation" at ClinicalTrials.gov
 |

