Chemistry:Orthoformic acid
From HandWiki
Short description: Hypothetical molecule with the formula HC(OH)3
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanetriol[1] | |
| Other names
Orthoformic acid
Trihydroxymethane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
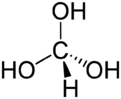
| HC(OH) 3 | |
| Molar mass | 64.040 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Orthoformic acid or methanetriol is a hypothetical chemical compound with the formula HC(OH)
3. In this molecule, the central carbon atom is bound to one hydrogen and three hydroxyl groups.
Orthoformic acid has not been isolated to date, and is believed to be unstable, decomposing into water and formic acid.[2]
Esters
Methanetriol esters, known as orthoformates, are well known and commercially available.[3][4] Like acetals, they are stable towards bases but easily hydrolyzed in acidic conditions to the alcohol and an ester of formic acid. They are used as mild dehydrating agents. Especially well known are trimethyl orthoformate, triethyl orthoformate, and triisopropyl orthoformate.
See also
- Methanol
- Methanediol
- Orthoacetic acid
- Orthocarbonic acid (methanetetrol)
References
- ↑ "Methanetriol - PubChem". NCBI. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5231666&loc=ec_rcs. Retrieved 22 April 2013.
- ↑ Böhm, S., Antipova, D. and Kuthan, J. (1996), "Study of methanetriol decomposition mechanisms". International Journal of Quantum Chemistry, volume 60, pages 649–655. doi:10.1002/(SICI)1097-461X(1996)60:2<649::AID-QUA3>3.0.CO;2-X
- ↑ Peter P. T. Sah, Tsu Sheng Ma (1932), ""ESTERS OF ORTHOFORMIC ACID". J. Am. Chem. Soc., volume 54, issue 7, pages 2964–2966 doi:10.1021/ja01346a048
- ↑ H. W. Post (1943), "The Chemistry of the Aliphatic Orthoesters", Reinhold, 188 pages
 |

