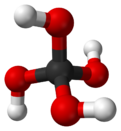
Chemistry:Orthocarbonic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanetetrol[1] | |||
| Systematic IUPAC name
Orthocarbonic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| CH4O4 | |||
| Molar mass | 80.039 g·mol−1 | ||
| Related compounds | |||
Other cations
|
|||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Orthocarbonic acid, carbon hydroxide or methanetetrol is the name given to a hypothetical compound with the chemical formula H
4CO
4 or C(OH)
4. Its molecular structure consists of a single carbon atom bonded to four hydroxy groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion orthocarbonate CO4−
4, and is therefore considered an oxoacid of carbon.
Orthocarbonic acid is highly unstable. Calculations show that it decomposes spontaneously into carbonic acid and water:[2][3]
Orthocarbonic acid is one of the group of ortho acids that have the general structure of RC(OH)
3.The term ortho acid is also used to refer to the most hydroxylated acid in a set of oxoacids.
Researchers predict that orthocarbonic acid is stable at high pressure; hence it may form in the interior of the ice giant planets Uranus and Neptune, where water and methane are common.[4]
Orthocarbonate anions
By loss of one through four protons, orthocarbonic acid could yield four anions: H
3CO−
4, H
2CO2−
4, HCO3−
4, and CO4−
4.
Numerous salts of fully deprotonated CO4−
4, such as Ca
2CO
4 or Sr
2CO
4, have been synthesized under high pressure conditions and structurally characterized by X-ray diffraction.[5][6][7] Strontium orthocarbonate, Sr
2CO
4, is stable at atmospheric pressure. Orthocarbonate is tetrahedral in shape, and is isoelectronic to orthonitrate. The C-O distance is 1.41 Å.[8] Sr
3[CO
4]O is an oxide orthocarbonate, also stable at atmospheric pressure.[9]
Orthocarbonate esters
The tetravalent moiety CO4 is found in stable organic compounds; they are formally esters of orthocarbonic acid, and therefore are called orthocarbonates. For example, tetraethoxymethane can be prepared by the reaction between chloropicrin and sodium ethoxide in ethanol.[10] Polyorthocarbonates are stable polymers that might have applications in absorbing organic solvents in waste treatment processes,[11] or in dental restorative materials.[12] The explosive trinitroethylorthocarbonate possesses an orthocarbonate core.
See also
- Pentaerythritol
- Silicic acid or Si(OH)4
- Carbonic acid or H2CO3
References
- ↑ "Methanetetrol - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=9547954&loc=ec_rcs.
- ↑ Bohm S.; Antipova D.; Kuthan J. (1997). "A Study of Methanetetraol Dehydration to Carbonic Acid". International Journal of Quantum Chemistry 62 (3): 315–322. doi:10.1002/(SICI)1097-461X(1997)62:3<315::AID-QUA10>3.0.CO;2-8.
- ↑ Carboxylic Acids and Derivatives IUPAC Recommendations on Organic & Biochemical Nomenclature
- ↑ G. Saleh; A. R. Oganov (2016). "Novel Stable Compounds in the C-H-O Ternary System at High Pressure". Scientific Reports 6: 32486. doi:10.1038/srep32486. PMID 27580525. Bibcode: 2016NatSR...632486S.
- ↑ Sagatova, Dinara; Shatskiy, Anton; Sagatov, Nursultan; Gavryushkin, Pavel N.; Litasov, Konstantin D. (2020). "Calcium orthocarbonate, Ca2CO4-Pnma: A potential host for subducting carbon in the transition zone and lower mantle". Lithos 370-371: 105637. doi:10.1016/j.lithos.2020.105637. ISSN 0024-4937. Bibcode: 2020Litho.37005637S.
- ↑ Binck, Jannes; Laniel, Dominique; Bayarjargal, Lkhamsuren; Khandarkhaeva, Saiana; Fedotenko, Timofey; Aslandukov, Andrey; Milman, Victor; Glazyrin, Konstantin et al. (2022). "Synthesis of calcium orthocarbonate, Ca2CO4-Pnma at P-T conditions of Earth's transition zone and lower mantle". American Mineralogist 107 (3): 336–342. doi:10.2138/am-2021-7872. Bibcode: 2022AmMin.107..336B. https://bib-pubdb1.desy.de/search?p=id:%22PUBDB-2022-02872%22.
- ↑ Laniel, Dominique; Binck, Jannes; Winkler, Björn; Vogel, Sebastian; Fedotenko, Timofey; Chariton, Stella; Prakapenka, Vitali; Milman, Victor et al. (2021). "Synthesis, crystal structure and structure–property relations of strontium orthocarbonate, Sr2CO4". Acta Crystallographica Section B 77 (1): 131–137. doi:10.1107/S2052520620016650. ISSN 2052-5206. Bibcode: 2021AcCrB..77..131L.
- ↑ Spahr, Dominik; Binck, Jannes; Bayarjargal, Lkhamsuren; Luchitskaia, Rita; Morgenroth, Wolfgang; Comboni, Davide; Milman, Victor; Winkler, Björn (4 April 2021). "Tetrahedrally Coordinated sp3-Hybridized Carbon in Sr2CO4 Orthocarbonate at Ambient Conditions". Inorganic Chemistry 60 (8): 5419–5422. doi:10.1021/acs.inorgchem.1c00159. PMID 33813824.
- ↑ Spahr, Dominik; König, Jannes; Bayarjargal, Lkhamsuren; Gavryushkin, Pavel N.; Milman, Victor; Liermann, Hanns-Peter; Winkler, Björn (4 October 2021). "Sr 3 [CO 4 ]O Antiperovskite with Tetrahedrally Coordinated sp 3 -Hybridized Carbon and OSr 6 Octahedra". Inorganic Chemistry 60 (19): 14504–14508. doi:10.1021/acs.inorgchem.1c01900. PMID 34520201.
- ↑ Orthocarbonic acid, tetraethyl ester Organic Syntheses, Coll. Vol. 4, p. 457 (1963); Vol. 32, p. 68 (1952).
- ↑ Sonmez, H.B.; Wudl, F. (2005). "Cross-linked poly(orthocarbonate)s as organic solvent sorbents". Macromolecules 38 (5): 1623–1626. doi:10.1021/ma048731x. Bibcode: 2005MaMol..38.1623S.
- ↑ Stansbury, J.W. (1992). "Synthesis and evaluation of new oxaspiro monomers for double ring-opening polymerization". Journal of Dental Research 71 (7): 1408–1412. doi:10.1177/00220345920710070901. PMID 1629456. http://jdr.iadrjournals.org/cgi/content/abstract/71/7/1408. Retrieved 2008-06-19.
 |