Chemistry:Osazone
From HandWiki

Osazone are a class of carbohydrate derivatives found in organic chemistry formed when reducing sugars are reacted with excess of phenylhydrazine at boiling temperatures.[1][2]
Formation
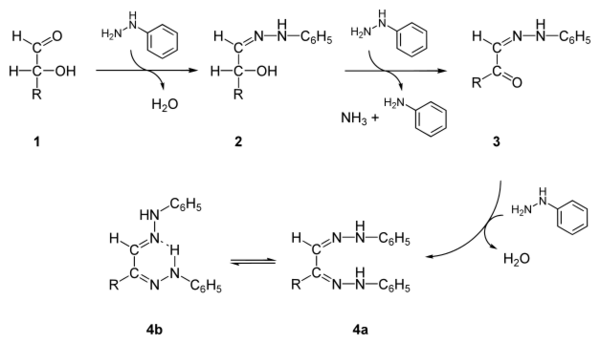
Osazone formation was developed by Emil Fischer,[3] who used the reaction as a test to identify monosaccharides.
The formation of a pair of hydrazone functionalities involves both oxidation and condensation reactions.[4] Since the reaction requires a free carbonyl group, only "reducing sugars" participate. Sucrose, which is nonreducing, does not form an osazone.
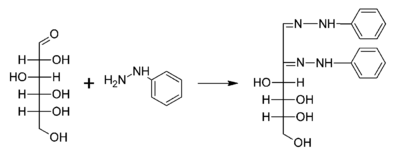
A typical reaction showing the formation of an osazone. D-glucose reacts with phenylhydrazine to give glucosazone. The same product is obtained from fructose and mannose.
Appearance
Osazones are highly coloured and crystalline compounds. Osazones are readily distinguished.[5]
- Maltosazone (from maltose) forms petal-shaped crystals.
- Lactosazone (from lactose) forms powder puff-shaped crystals.
- Galactosazone (from galactose) forms rhombic-plate shaped crystals.
- Glucosazone (from glucose, fructose or mannose) forms broomstick or needle-shaped crystals.
Historic references
- Fischer, Emil (1908). "Schmelzpunkt des Phenylhydrazins und einiger Osazone". Berichte der Deutschen Chemischen Gesellschaft 41: 73–77. doi:10.1002/cber.19080410120. https://zenodo.org/record/1426269.
- Fischer, Emil (1894). "Ueber einige Osazone und Hydrazone der Zuckergruppe". Berichte der Deutschen Chemischen Gesellschaft 27 (2): 2486–2492. doi:10.1002/cber.189402702249. https://zenodo.org/record/1425750.
- Barry, VINCENT C.; Mitchell, PW (1955). "Mechanism of Osazone Formation". Nature 175 (4448): 220. doi:10.1038/175220a0. PMID 13235861. Bibcode: 1955Natur.175..220B.
References
- ↑ El Khadem, Hassan S.; Fatiadi, Alexander J. (2000). "Hydrazine derivatives of carbohydrates and related compounds". Advances in Carbohydrate Chemistry and Biochemistry 55: 175-263. doi:10.1016/S0065-2318(00)55006-9. ISBN 9780120072552. https://www.sciencedirect.com/science/article/abs/pii/S0065231800550069. Retrieved 31 March 2021.
- ↑ Mester, L.; El Khadem, H.; Horton, D. (1970). "Structure of saccharide osazones". Journal of the Chemical Society C: Organic (18): 2567. doi:10.1039/J39700002567.
- ↑ Helferich, B. (1953). "Emil Fischer zum 100. Geburtstag". Angewandte Chemie 65 (2): 45–52. doi:10.1002/ange.19530650202. Bibcode: 1953AngCh..65...45H.
- ↑ Ramakrishnan, S. (2004) (in en). Textbook of Medical Biochemistry. Orient Blackswan. ISBN 9788125020714. https://books.google.com/books?id=BVpDI7n2M9gC&pg=PA20.
- ↑ Gupta, Anil (2019). "Carbohydrates". Comprehensive Biochemistry for Dentistry. Singapore: Springer. pp. 108–110. ISBN 978-981-13-1035-5.
 |