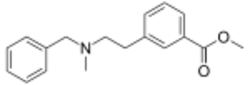
Chemistry:PRL-8-53
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H21NO2 |
| Molar mass | 283.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
PRL-8-53 is a nootropic substituted phenethylamine that has been shown to act as a hypermnesic drug in humans; it was first synthesized by medical chemistry professor Nikolaus Hansl at Creighton University in the 1970s as part of his work on amino ethyl meta benzoic acid esters.[1][2]
Nootropic effects
PRL-8-53 is not a recognized treatment for any disease or disorder, although a nootropic effect has been claimed in healthy individuals. A single double-blind trial exploring the effects of PRL-8-53 in 47 healthy volunteers on various cognitive tasks remains the only human trial of PRL-8-53 to date. In the study, 5 mg of the drug was administered orally 2–2.5 hours before study tasks.[1] Overall improvements in recollection differed between subject groups when compared to their respective placebo scores. Poorer performers (six words or fewer) showed an 87.5-105% increase in recollection while higher performers (eight or more words) exhibited a smaller 7.9-14% increase which failed to reach statistical significance. Older subjects (<30 years old) experienced an average 108-152% improvement in recollection. The researchers noted that this was likely a result of a ceiling effect due to many study participants scoring close to 100% on the recall test even on placebo.[1] No side effects were reported during the trial.[1]
Mechanism of action
The exact mechanism of action of PRL-8-53 remains unknown. Doses up to 200 mg/kg are not observed to have stimulant properties, and a dosage of 20 mg/kg does not potentiate the effects of dextroamphetamine in rats.[1] It displays possible cholinergic properties, and potentiates dopamine while partially inhibiting serotonin.[1] PRL-8-53 reverses the catatonic and ptotic effects of reserpine.[1][3]
Toxicity
PRL-8-53 is relatively non-toxic, with an oral -1">50 in mice of 860 mg/kg, giving the drug a high therapeutic index. Doses above 8 mg/kg have brief hypotensive effects in canines. High doses depress motor activity in the rat and mouse, with the ED50 for a 50% reduction in motor activity of mice at 160 mg/kg. PRL-8-53 displays spasmolytic effects.[3]
Reasons for discontinuation
It is uncertain as to exactly why PRL-8-53's development was halted. PRL-8-53 did not exhibit obvious side effects that would warrant discontinuation in animals or in its single human trial. Factors not limited to the retirement of Dr. Nikolaus Hansl in 1985 and a lawsuit filed between Hansl and Creighton University may be to blame.
Hansl v. Creighton University
On July 1, 1985 Dr. Nikolaus Hansl entered into a settlement agreement with Creighton University.[4] The terms of the agreement guaranteed Dr. Hansl access to his laboratory facilities and office space at the university for a period of 2 years.[4] Upon returning from a 4-month absence. Hansl found that the refrigerator he had used to store various experimental compounds had been unplugged, purportedly rendering the compounds unusable.[4]
It is uncertain if samples of PRL-8-53 were stored in the fridge or if they would have been degraded significantly under room temperature conditions.
Patent
Dr. Hansl's patent for the synthesis of PRL-8-53 has expired.[5]
Synonyms
Methyl 3-(2-(benzylmethylamino)ethyl)benzoate hydrochloride
3-(2-benzylmethylaminoethyl) benzoic acid methyl ester hydrochloride
3-(2-(Methyl(phenylmethyl)amino)ethyl)benzoic acid methyl ester hydrochloride
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "PRL-8-53: enhanced learning and subsequent retention in humans as a result of low oral doses of new psychotropic agent". Psychopharmacology 56 (3): 249–53. April 1978. doi:10.1007/BF00432846. PMID 418433.
- ↑ US Patent 3870715 A: Substituted amino ethyl meta benzoic acid esters
- ↑ 3.0 3.1 "A novel spasmolytic and CNS active agent: 3-(2-benzylmethylamino ethyl) benzoic acid methyl ester hydrochloride". Experientia 30 (3): 271–2. March 1974. doi:10.1007/BF01934822. PMID 4824605.
- ↑ 4.0 4.1 4.2 "Hansl v. Creighton University" (in en). https://law.justia.com/cases/nebraska/supreme-court/1993/1066-0.html.
- ↑ Nikolaus R. Hansl; Process for hydrolyzing 3-trifluoromethyl phenethylamines (US patent 3 792 048) 1974
 |

