Chemistry:Dextroamphetamine
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɛkstroʊæmˈfɛtəmiːn/ |
| Trade names | Dexedrine, Dextrostat, Xelstrym, Zenzedi, others |
| Other names | d-Amphetamine, (S)-Amphetamine, S(+)-Amphetamine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605027 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Moderate[1][2] - high[3][4][5] |
| Addiction liability | Moderate[1][2] - high[3][4][5] |
| Routes of administration | By mouth, transdermal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 75–100%[11] |
| Protein binding | 15–40%[12] |
| Metabolism | CYP2D6,[17] DBH,[23] FMO3[24] |
| Onset of action | IR dosing: 0.5–1.5 hours[13][14] XR dosing: 1.5–2 hours[15][16] |
| Elimination half-life | 9–11 hours[17][18] pH-dependent: 7–34 hours[19] |
| Duration of action | IR dosing: 3–6 hours[15][20] XR dosing: 8–12 hours[21][15][20] |
| Excretion | Kidney (45%);[22] urinary pH-dependent |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H13N |
| Molar mass | 135.210 g·mol−1 |
| 3D model (JSmol) | |
| Density | 0.913 g/cm3 |
| Boiling point | 201.5 °C (394.7 °F) |
| Solubility in water | 20 |
| |
| |
| | |
Dextroamphetamine is a potent central nervous system (CNS) stimulant and enantiomer[note 1] of amphetamine that is prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[8][25] It is also used as an athletic performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant.
The amphetamine molecule exists as two enantiomers,[note 1] levoamphetamine and dextroamphetamine. Dextroamphetamine is the dextrorotatory, or 'right-handed', enantiomer and exhibits more pronounced effects on the central nervous system than levoamphetamine. Pharmaceutical dextroamphetamine sulfate is available as both a brand name and generic drug in a variety of dosage forms. Dextroamphetamine is sometimes prescribed as the inactive prodrug lisdexamfetamine dimesylate, which is converted into dextroamphetamine after absorption.
Dextroamphetamine, like other amphetamines, elicits its stimulating effects via several distinct actions: it inhibits or reverses the transporter proteins for the monoamine neurotransmitters (namely the serotonin, norepinephrine and dopamine transporters) either via trace amine-associated receptor 1 (TAAR1) or in a TAAR1 independent fashion when there are high cytosolic concentrations of the monoamine neurotransmitters[27] and it releases these neurotransmitters from synaptic vesicles via vesicular monoamine transporter 2.[28] It also shares many chemical and pharmacological properties with human trace amines, particularly phenethylamine and N-methylphenethylamine, the latter being an isomer of amphetamine produced within the human body.
Uses
Medical

Dextroamphetamine is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder),[8] and is sometimes prescribed off-label for depression and obesity.[25] {{#section-h:Amphetamine|Medical}}
Enhancing performance
{{#section-h:Amphetamine|Enhancing performance}}
Recreational
Dextroamphetamine is also used recreationally as a euphoriant and aphrodisiac, and like other amphetamines is used as a club drug for its energetic and euphoric high. Dextroamphetamine is considered to have a high potential for misuse in a recreational manner since individuals typically report feeling euphoric, more alert, and more energetic after taking the drug.[29][30][31] Dextroamphetamine's dopaminergic (rewarding) properties affect the mesocorticolimbic circuit; a group of neural structures responsible for incentive salience (i.e., "wanting"; desire or craving for a reward and motivation), positive reinforcement and positively-valenced emotions, particularly ones involving pleasure.[32] Large recreational doses of dextroamphetamine may produce symptoms of dextroamphetamine overdose.[31] Recreational users sometimes open dexedrine capsules and crush the contents in order to insufflate (snort) it or subsequently dissolve it in water and inject it.[31] Immediate-release formulations have higher potential for abuse via insufflation (snorting) or intravenous injection due to a more favorable pharmacokinetic profile and easy crushability (especially tablets).[33][34]
The reason for using crushed spansules for insufflation and injection methods is evidently due to the "instant-release" forms of the drug seen in tablet preparations often containing a sizable amount of inactive binders and fillers alongside the active d-amphetamine, such as dextrose.[35] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[31] Chronic overuse of dextroamphetamine can lead to severe drug dependence, resulting in withdrawal symptoms when drug use stops.[31]
Contraindications
{{#section-h:Amphetamine|Contraindications}}
Adverse effects
{{#section-h:Amphetamine|Adverse effects}}
Overdose
{{#section-h:Amphetamine|Overdose}}
Interactions
Many types of substances are known to interact with amphetamine, resulting in altered drug action or metabolism of amphetamine, the interacting substance, or both.[17][36][37] Inhibitors of the enzymes that metabolize amphetamine (e.g., CYP2D6 and FMO3) will prolong its elimination half-life, meaning that its effects will last longer.[24][36][37] Amphetamine also interacts with MAOIs, particularly monoamine oxidase A inhibitors, since both MAOIs and amphetamine increase plasma catecholamines (i.e., norepinephrine and dopamine);[36][37] therefore, concurrent use of both is dangerous.[36][37] Amphetamine modulates the activity of most psychoactive drugs. In particular, amphetamine may decrease the effects of sedatives and depressants and increase the effects of stimulants and antidepressants.[36][37] Amphetamine may also decrease the effects of antihypertensives and antipsychotics due to its effects on blood pressure and dopamine respectively.[36][37] Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 2][41]
Pharmacology
Pharmacodynamics
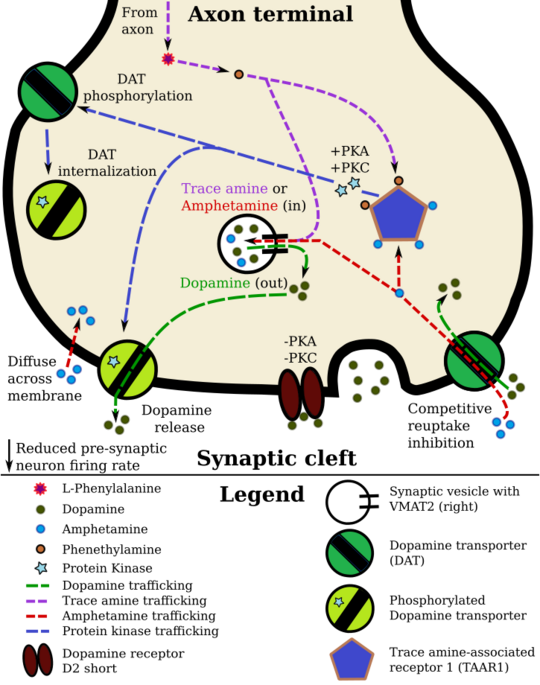
Pharmacodynamics of amphetamine in a dopamine neuron
|
Amphetamine and its enantiomers have been identified as potent full agonists of trace amine-associated receptor 1 (TAAR1), a GPCR, discovered in 2001, that is important for regulation of monoaminergic systems in the brain.[47][48] Activation of TAAR1 increases cAMP production via adenylyl cyclase activation and inhibits the function of the dopamine transporter, norepinephrine transporter, and serotonin transporter, as well as inducing the release of these monoamine neurotransmitters (effluxion).[27][47][49] Amphetamine enantiomers are also substrates for a specific neuronal synaptic vesicle uptake transporter called VMAT2.[28] When amphetamine is taken up by VMAT2, the vesicle releases (effluxes) dopamine, norepinephrine, and serotonin, among other monoamines, into the cytosol in exchange.[28]
Dextroamphetamine (the dextrorotary enantiomer) and levoamphetamine (the levorotary enantiomer) have identical pharmacodynamics, but their binding affinities to their biomolecular targets vary.[48][50] Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[48] Consequently, dextroamphetamine produces roughly three to four times more central nervous system (CNS) stimulation than levoamphetamine;[48][50] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[50]
Related endogenous compounds
{{#section-h:Amphetamine|Related endogenous compounds}}
Pharmacokinetics
{{#section-h:Amphetamine|Pharmacokinetics}}
History, society, and culture
Racemic amphetamine was first synthesized under the chemical name "phenylisopropylamine" in Berlin, 1887 by the Romanian chemist Lazăr Edeleanu. It was not widely marketed until 1932, when the pharmaceutical company Smith, Kline & French (now known as GlaxoSmithKline) introduced it in the form of the Benzedrine inhaler for use as a bronchodilator. Notably, the amphetamine contained in the Benzedrine inhaler was the liquid free-base,[note 3] not a chloride or sulfate salt.
Three years later, in 1935, the medical community became aware of the stimulant properties of amphetamine, specifically the dextroamphetamine isomer, and in 1937 Smith, Kline, and French introduced tablets under the brand name Dexedrine.[51] In the United States, Dexedrine was approved to treat narcolepsy and attention disorders.[8] In Canada indications once included epilepsy and parkinsonism.[52] Dextroamphetamine was marketed in various other forms in the following decades, primarily by Smith, Kline, and French, such as several combination medications including a mixture of dextroamphetamine and amobarbital (a barbiturate) sold under the tradename Dexamyl and, in the 1950s, an extended release capsule (the "Spansule").[53] Preparations containing dextroamphetamine were also used in World War II as a treatment against fatigue.[54]
It quickly became apparent that dextroamphetamine and other amphetamines had a high potential for misuse, although they were not heavily controlled until 1970, when the Comprehensive Drug Abuse Prevention and Control Act was passed by the United States Congress. Dextroamphetamine, along with other sympathomimetics, was eventually classified as Schedule II, the most restrictive category possible for a drug with a government-sanctioned, recognized medical use.[55] Internationally, it has been available under the names AmfeDyn (Italy), Curban (US), Obetrol (Switzerland), Simpamina (Italy), Dexedrine/GSK (US & Canada), Dexedrine/UCB (United Kingdom), Dextropa (Portugal), and Stild (Spain).[56] It became popular on the mod scene in England in the early 1960s, and carried through to the Northern Soul scene in the north of England to the end of the 1970s.
In October 2010, GlaxoSmithKline sold the rights for Dexedrine Spansule to Amedra Pharmaceuticals (a subsidiary of CorePharma).[57]
The U.S. Air Force uses dextroamphetamine as one of its "go pills", given to pilots on long missions to help them remain focused and alert. Conversely, "no-go pills" are used after the mission is completed, to combat the effects of the mission and "go-pills".[58][59][60] The Tarnak Farm incident was linked by media reports to the use of this drug on long term fatigued pilots. The military did not accept this explanation, citing the lack of similar incidents. Newer stimulant medications or awakeness promoting agents with different side effect profiles, such as modafinil, are being investigated and sometimes issued for this reason.[59]
Formulations
| Brand name |
United States Adopted Name |
(D:L) ratio | Dosage form |
Marketing start date |
Sources |
|---|---|---|---|---|---|
| Adderall | Mixed amphetamine salts | 3:1 (salts) | tablet | 1996 | [25][69] |
| Adderall XR | Mixed amphetamine salts | 3:1 (salts) | capsule | 2001 | [25][69] |
| Mydayis | Mixed amphetamine salts | 3:1 (salts) | capsule | 2017 | [70] |
| Adzenys XR-ODT | amphetamine | 3:1 (base) | ODT | 2016 | [71][72] |
| Dyanavel XR | amphetamine | 3.2:1 (base) | suspension | 2015 | [73][74] |
| Evekeo | amphetamine sulfate | 1:1 (salts) | tablet | 2012 | [75] [76] |
| Dexedrine | dextroamphetamine sulfate | 1:0 (salts) | capsule | 1976 | [25][69] |
| Zenzedi | dextroamphetamine sulfate | 1:0 (salts) | tablet | 2013 | [69] |
| Vyvanse | lisdexamfetamine dimesylate | 1:0 (prodrug) | capsule | 2007 | [25][77] |
| tablet | |||||
| Xelstrym | dextroamphetamine | 1:0 (base) | patch | 2022 | [9] |
Transdermal Dextroamphetamine Patches
Dextroamphetamine is available as a transdermal patch containing dextroamphetamine base under the brand name Xelstrym.[9]
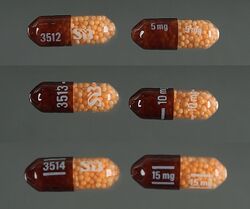
Dextroamphetamine sulfate

In the United States, immediate release (IR) formulations of dextroamphetamine sulfate are available generically as 5 mg and 10 mg tablets, marketed by Barr (Teva Pharmaceutical Industries), Mallinckrodt Pharmaceuticals, Wilshire Pharmaceuticals, Aurobindo Pharmaceutical USA and CorePharma. Previous IR tablets sold under the brand names Dexedrine and Dextrostat have been discontinued but in 2015, IR tablets became available by the brand name Zenzedi, offered as 2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg and 30 mg tablets.[78] Dextroamphetamine sulfate is also available as a controlled-release (CR) capsule preparation in strengths of 5 mg, 10 mg, and 15 mg under the brand name Dexedrine Spansule, with generic versions marketed by Barr and Mallinckrodt. A bubblegum flavored oral solution is available under the brand name ProCentra, manufactured by FSC Pediatrics, which is designed to be an easier method of administration in children who have difficulty swallowing tablets, each 5 mL contains 5 mg dextroamphetamine.[79] The conversion rate between dextroamphetamine sulfate to amphetamine free base is .728.[80]
In Australia, dexamfetamine is available in bottles of 100 instant release 5 mg tablets as a generic drug[81] or slow release dextroamphetamine preparations may be compounded by individual chemists.[82] In the United Kingdom, it is available in 5 mg instant release sulfate tablets under the generic name dexamfetamine sulfate as well as 10 mg and 20 mg strength tablets under the brand name Amfexa. It is also available in generic dexamfetamine sulfate 5 mg/ml oral sugar-free syrup.[83] The brand name Dexedrine was available in the United Kingdom prior to UCB Pharma disinvesting the product to another pharmaceutical company (Auden Mckenzie).[84]
Lisdexamfetamine
Dextroamphetamine is the active metabolite of the prodrug lisdexamfetamine (L-lysine-dextroamphetamine), available by the brand name Vyvanse (Elvanse in the European market) (Venvanse in the Brazil market) (lisdexamfetamine dimesylate). Dextroamphetamine is liberated from lisdexamfetamine enzymatically following contact with red blood cells. The conversion is rate-limited by the enzyme, which prevents high blood concentrations of dextroamphetamine and reduces lisdexamfetamine's drug liking and abuse potential at clinical doses.[85][86] Vyvanse is marketed as once-a-day dosing as it provides a slow release of dextroamphetamine into the body. Vyvanse is available as capsules, and chewable tablets, and in seven strengths; 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg. The conversion rate between lisdexamfetamine dimesylate (Vyvanse) to dextroamphetamine base is 29.5%.[87][88][89]
Adderall

Another pharmaceutical that contains dextroamphetamine is commonly known by the brand name Adderall.[36][37] It is available as immediate release (IR) tablets and extended release (XR) capsules.[36][37] Adderall contains equal amounts of four amphetamine salts:[36][37]
- One-quarter racemic (d,l-)amphetamine aspartate monohydrate
- One-quarter dextroamphetamine saccharate
- One-quarter dextroamphetamine sulfate
- One-quarter racemic (d,l-)amphetamine sulfate
Adderall has a total amphetamine base equivalence of 63%.[36][37] While the enantiomer ratio by dextroamphetamine salts to levoamphetamine salts is 3:1, the amphetamine base content is 75.9% dextroamphetamine, 24.1% levoamphetamine. [note 5]
| drug | formula | molecular mass [note 6] |
amphetamine base [note 7] |
amphetamine base in equal doses |
doses with equal base content [note 8] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[91][92] | (C9H13N)2•H2SO4 | |||||||||
| amphetamine sulfate[93] | (C9H13N)2•H2SO4 | |||||||||
| Adderall | ||||||||||
| 25% | dextroamphetamine sulfate[91][92] | (C9H13N)2•H2SO4 | ||||||||
| 25% | amphetamine sulfate[93] | (C9H13N)2•H2SO4 | ||||||||
| 25% | dextroamphetamine saccharate[94] | (C9H13N)2•C6H10O8 | ||||||||
| 25% | amphetamine aspartate monohydrate[95] | (C9H13N)•C4H7NO4•H2O | ||||||||
| lisdexamfetamine dimesylate[77] | C15H25N3O•(CH4O3S)2 | |||||||||
| amphetamine base suspension[note 9][73] | C9H13N | |||||||||
Notes
- ↑ 1.0 1.1 Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.[26]
- ↑ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[38][39][40] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[40]
- ↑ Free-base form amphetamine is a volatile oil, hence the efficacy of the inhalers.
- ↑ These represent the current brands in the United States, except Dexedrine instant release tablets. Dexedrine tablets, introduced in 1937, is discontinued but available as Zenzedi and generically;[61][62] Dexedrine listed here represents the extended release "Spansule" capsule which was approved in 1976.[63][64] Amphetamine sulfate tablets, now sold as Evekeo (brand), were originally sold as Benzedrine (brand) sulfate in 1935[65][66] and discontinued sometime after 1982.[67][68]
- ↑ Calculated by dextroamphetamine base percent / total amphetamine base percent = 47.49/62.57 = 75.90% from table: Amphetamine base in marketed amphetamine medications. The remainder is levoamphetamine.
- ↑ For uniformity, molecular masses were calculated using the Lenntech Molecular Weight Calculator[90] and were within 0.01g/mol of published pharmaceutical values.
- ↑ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ↑ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- ↑ This product (Dyanavel XR) is an oral suspension (i.e., a drug that is suspended in a liquid and taken by mouth) that contains 2.5 mg/mL of amphetamine base.[73] The product uses an ion exchange resin to achieve extended release of the amphetamine base.[73]
- Image legend
Reference notes
References
- ↑ 1.0 1.1 "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child and Adolescent Psychiatric Clinics of North America 17 (2): 459–74, xi. April 2008. doi:10.1016/j.chc.2007.11.010. PMID 18295156.
- ↑ 2.0 2.1 "European guidelines on managing adverse effects of medication for ADHD". European Child & Adolescent Psychiatry 20 (1): 17–37. January 2011. doi:10.1007/s00787-010-0140-6. PMID 21042924.
- ↑ 3.0 3.1 "Evaluation of risks associated with short- and long-term psychostimulant therapy for treatment of ADHD in children". Expert Opinion on Drug Safety 3 (2): 93–100. March 2004. doi:10.1517/14740338.3.2.93. PMID 15006715.
- ↑ 4.0 4.1 "The potential for misuse and abuse of medications in ADHD: a review". Postgraduate Medicine 126 (5): 64–81. September 2014. doi:10.3810/pgm.2014.09.2801. PMID 25295651.
- ↑ 5.0 5.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedStahl's Essential Psychopharmacology - ↑ "Therapeutic Goods (Poisons Standard—February 2023) Instrument 2022". https://www.legislation.gov.au/Series/F2022L01257.
- ↑ "ADHD Stimulant Prescribing Regulations & Authorities in Australia & New Zealand". 20 February 2022. https://aadpa.com.au/adhd-stimulant-prescribing-regulations-in-australia-new-zealand/.
- ↑ 8.0 8.1 8.2 8.3 "Dexedrine spansule- dextroamphetamine sulfate capsule, extended release". 10 January 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc717b9b-22ea-4c60-a1d4-ee38a40bce78.
- ↑ 9.0 9.1 9.2 "Xelstrym- dextroamphetamine patch, extended release". 6 January 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0862f02a-72a8-41cc-8845-57cf4974bb6f.
- ↑ "List of nationally authorised medicinal products : Active substance(s): dexamfetamine : Procedure No. PSUSA/00000986/202109". https://www.ema.europa.eu/documents/psusa/dexamfetamine-list-nationally-authorised-medicinal-products-psusa/00000986/202109_en.pdf.
- ↑ "Dextromphetamine". Dextromphetamine. http://www.drugbank.ca/drugs/DB01576#pharmacology. Retrieved 5 November 2013.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDrugbank-amph - ↑ Primary Care Pediatrics. Lippincott Williams & Wilkins. 1 January 2001. p. 243. ISBN 9780781720083. https://books.google.com/books?id=o43u_qWT4asC.|quote = Table 21.2 Medications for ADHD ... D-amphetamine ... Onset: 30 min.
- ↑ "Dexedrine, ProCentra(dextroamphetamine) dosing, indications, interactions, adverse effects, and more". http://reference.medscape.com/drug/dexedrine-procentra-dextroamphetamine-342998#10. "Onset of action: 1–1.5 hr"
- ↑ 15.0 15.1 15.2 Millichap JG, ed (2010). "Chapter 9: Medications for ADHD". Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. p. 112. ISBN 9781441913968. "
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h]" - ↑ "Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder". Postgrad. Med. 120 (3): 69–88. September 2008. doi:10.3810/pgm.2008.09.1909. PMID 18824827. "Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. ... MAS-XR, and LDX have a long duration of action at 12 hours postdose".
- ↑ 17.0 17.1 17.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedFDA Pharmacokinetics - ↑ "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". 27 February 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f22635fe-821d-4cde-aa12-419f8b53db81.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedHSDB Toxnet October 2017 Full archived record - ↑ 20.0 20.1 "A practical guide to the therapy of narcolepsy and hypersomnia syndromes". Neurotherapeutics 9 (4): 739–752. October 2012. doi:10.1007/s13311-012-0150-9. PMID 23065655.
- ↑ "Amphetamine (D)". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. March 2017. pp. 39–44. ISBN 9781108228749. https://books.google.com/books?id=9hssDwAAQBAJ&pg=PA39. Retrieved 8 August 2017.
- ↑ "dextrostat (dextroamphetamine sulfate) tablet [Shire US Inc."]. Wayne, PA: Shire US Inc.. August 2006. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=1645.
- ↑ Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2013. p. 648. ISBN 978-1609133450. "Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine."
- ↑ 24.0 24.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedFMO - ↑ 25.0 25.1 25.2 25.3 25.4 25.5 "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. June 2013. doi:10.1177/0269881113482532. PMID 23539642.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomer". doi:10.1351/goldbook.E02069
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry 116 (2): 164–176. January 2011. doi:10.1111/j.1471-4159.2010.07109.x. PMID 21073468.
- ↑ 28.0 28.1 28.2 28.3 28.4 "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Annals of the New York Academy of Sciences 1216 (1): 86–98. January 2011. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013. Bibcode: 2011NYASA1216...86E.
- ↑ "Commonly Abused Prescription Drugs Chart". National Institute on Drug Abuse. http://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs/commonly-abused-prescription-drugs-chart.
- ↑ "Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. http://www.drugabuse.gov/publications/infofacts/stimulant-adhd-medications-methylphenidate-amphetamines.
- ↑ 31.0 31.1 31.2 31.3 31.4 "National Institute on Drug Abuse. 2009. Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. http://www.drugabuse.gov/publications/drugfacts/stimulant-adhd-medications-methylphenidate-amphetamines.
- ↑ "Neuronal reward and decision signals: from theories to data". Physiological Reviews 95 (3): 853–951. 2015. doi:10.1152/physrev.00023.2014. PMID 26109341. "Rewards in operant conditioning are positive reinforcers. ... Operant behavior gives a good definition for rewards. Anything that makes an individual come back for more is a positive reinforcer and therefore a reward. Although it provides a good definition, positive reinforcement is only one of several reward functions. ... Rewards are attractive. They are motivating and make us exert an effort. ... Rewards induce approach behavior, also called appetitive or preparatory behavior, sexual behavior, and consummatory behavior. ... Thus any stimulus, object, event, activity, or situation that has the potential to make us approach and consume it is by definition a reward. ... Rewarding stimuli, objects, events, situations, and activities consist of several major components. First, rewards have basic sensory components (visual, auditory, somatosensory, gustatory, and olfactory) ... Second, rewards are salient and thus elicit attention, which are manifested as orienting responses. The salience of rewards derives from three principal factors, namely, their physical intensity and impact (physical salience), their novelty and surprise (novelty/surprise salience), and their general motivational impact shared with punishers (motivational salience). A separate form not included in this scheme, incentive salience, primarily addresses dopamine function in addiction and refers only to approach behavior (as opposed to learning) ... Third, rewards have a value component that determines the positively motivating effects of rewards and is not contained in, nor explained by, the sensory and attentional components. This component reflects behavioral preferences and thus is subjective and only partially determined by physical parameters. Only this component constitutes what we understand as a reward. It mediates the specific behavioral reinforcing, approach generating, and emotional effects of rewards that are crucial for the organism's survival and reproduction, whereas all other components are only supportive of these functions. ... Rewards can also be intrinsic to behavior. They contrast with extrinsic rewards that provide motivation for behavior and constitute the essence of operant behavior in laboratory tests. Intrinsic rewards are activities that are pleasurable on their own and are undertaken for their own sake, without being the means for getting extrinsic rewards. ... Intrinsic rewards are genuine rewards in their own right, as they induce learning, approach, and pleasure, like perfectioning, playing, and enjoying the piano. Although they can serve to condition higher order rewards, they are not conditioned, higher order rewards, as attaining their reward properties does not require pairing with an unconditioned reward. ... These emotions are also called liking (for pleasure) and wanting (for desire) in addiction research and strongly support the learning and approach generating functions of reward.".
- ↑ Canadian ADHD Practice Guidelines (Fourth ed.). Canadian ADHD Resource Alliance. 2018. p. 67. https://www.caddra.ca/wp-content/uploads/CADDRA-Guidelines-4th-Edition_-Feb2018.pdf. Retrieved 2 May 2023.
- ↑ "Abuse of medications employed for the treatment of ADHD: results from a large-scale community survey". Medscape Journal of Medicine 10 (5): 111. May 2008. PMID 18596945.
- ↑ "Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans". Addict Biology 18 (6): 985–992. November 2013. doi:10.1111/j.1369-1600.2011.00416.x. PMID 22129527.
- ↑ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". 27 February 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f22635fe-821d-4cde-aa12-419f8b53db81.
- ↑ 37.00 37.01 37.02 37.03 37.04 37.05 37.06 37.07 37.08 37.09 "Adderall XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release". 3 March 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aff45863-ffe1-4d4f-8acf-c7081512a6c0.
- ↑ "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Rev. Neurother. 8 (4): 611–625. April 2008. doi:10.1586/14737175.8.4.611. PMID 18416663. "Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.".
- ↑ "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron 69 (4): 628–649. February 2011. doi:10.1016/j.neuron.2011.02.010. PMID 21338876. "They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).".
- ↑ 40.0 40.1 "The role of zinc ions in reverse transport mediated by monoamine transporters". J. Biol. Chem. 277 (24): 21505–21513. June 2002. doi:10.1074/jbc.M112265200. PMID 11940571.
- ↑ "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". J. Am. Acad. Child Adolesc. Psychiatry 51 (10): 1003–1019.e20. October 2012. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
- ↑ "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia 6 (3): 123–148. August 2016. doi:10.1016/j.baga.2016.02.001. PMID 27141430. "Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.".
- ↑ "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. July 2011. doi:10.3389/fnsys.2011.00056. PMID 21772817. "Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.".
- ↑ "TAAR1". GenAtlas. University of Paris. 28 January 2012. http://genatlas.medecine.univ-paris5.fr/fiche.php?symbol=TAAR1. Retrieved 29 May 2014. " • tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)"
- ↑ "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron 83 (2): 404–416. July 2014. doi:10.1016/j.neuron.2014.05.043. PMID 25033183. "AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH".
- ↑ "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. September 2013. doi:10.1016/j.tips.2013.07.005. PMID 23968642. "AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].".
- ↑ 47.0 47.1 "Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor". Molecular Pharmacology 60 (6): 1181–1188. December 2001. doi:10.1124/mol.60.6.1181. PMID 11723224.
- ↑ 48.0 48.1 48.2 48.3 "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. December 2011. doi:10.1016/j.bmc.2011.10.007. PMID 22037049.
- ↑ "Trace amines: identification of a family of mammalian G protein-coupled receptors". Proceedings of the National Academy of Sciences of the United States of America 98 (16): 8966–8971. July 2001. doi:10.1073/pnas.151105198. PMID 11459929. Bibcode: 2001PNAS...98.8966B.
- ↑ 50.0 50.1 50.2 Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. 2010. ISBN 9780071624428.
- ↑ "Dexedrine". http://www.medic8.com/medicines/Dexedrine.html.
- ↑ "Dextroamphetamine [monograph"]. http://www.mentalhealth.com/drug/p30-d04.html.
- ↑ "Information on Dexedrine: A Quick Review | Weitz & Luxenberg". Weitzlux.com. 31 August 2013. http://www.weitzlux.com/Dexedrine/information_403484.html.
- ↑ "Amphetamine, past and present—a pharmacological and clinical perspective". Journal of Psychopharmacology 27 (6): 479–96. June 2013. doi:10.1177/0269881113482532. PMID 23539642.
- ↑ "Prescription Forgery". Handwriting Services International. 4 January 2017. http://www.denton.handwritingexperts.com/articles/prescriptionforgery1.html.
- ↑ Pharmaceutical Manufacturing Encyclopedia. 1 (2nd ed.). Noyes Publications. ISBN 978-0-8155-1144-1. http://www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPLAY_bookid=598.
- ↑ "Dexedrine FAQs". http://www.dexedrine.net/faq.asp.
- ↑ "'Go pills': A war on drugs?". NBC News. 9 January 2003. http://www.nbcnews.com/id/3071789/ns/us_news-only/t/go-pills-war-drugs/.
- ↑ 59.0 59.1 "Air Force scientists battle aviator fatigue". http://www.af.mil/news/story.asp?id=123007615.
- ↑ "The use of amphetamines in U.S. Air Force tactical operations during Desert Shield and Storm". Aviation, Space, and Environmental Medicine 66 (3): 260–3. 1995. PMID 7661838.
- ↑ "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology 27 (6): 479–496. June 2013. doi:10.1177/0269881113482532. PMID 23539642. "Smith, Kline and French synthesised both isomers, and in 1937 commenced marketing of d-amphetamine, which was the more potent of the two isomers, under the trade name of Dexedrine.".
- ↑ "Drugs@FDA: Dexedrine". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process.
- ↑ "Drugs@FDA: Dexedrine". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=017078.
- ↑ "Drugs@FDA: Dexedrine: Label and Approval History". http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Set_Current_Drug&ApplNo=017078&DrugName=DEXEDRINE&ActiveIngred=DEXTROAMPHETAMINE%2520SULFATE&SponsorApplicant=AMEDRA%2520PHARMS&ProductMktStatus=1&goto=Search.Label_ApprovalHistory. "08/02/1976 ... Approval"
- ↑ "Bradley's Benzedrine studies on children with behavioral disorders". The Yale Journal of Biology and Medicine 84 (1): 27–33. March 2011. PMID 21451781. "Bradley experimented with Benzedrine sulfate, a drug marketed to doctors by the company Smith, Kline & French (SKF) between 1935 and 1937...".
- ↑ "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology 27 (6): 479–496. June 2013. doi:10.1177/0269881113482532. PMID 23539642. "Smith, Kline and French introduced Benzedrine onto the market in 1935 as a treatment for narcolepsy (for which it is still used today), mild depression, post-encephalitic Parkinsonism and a raft of other disorders.".
- ↑ "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology 27 (6): 479–496. June 2013. doi:10.1177/0269881113482532. PMID 23539642. "The use of Benzedrine to treat ADHD declined dramatically after Gross (1976) reported that the racemate was significantly less clinically effective than Dexedrine. Currently, the only use of l-amphetamine in ADHD medications is in mixed salts/mixed enantiomers amphetamine...".
- ↑ "FDA Approved Drug Products: Label and Approval History (Benzedrine)". http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. "Action Date 5/11/1982, Supplement Number 007, Approval Type Chemistry"
- ↑ 69.0 69.1 69.2 69.3 "National Drug Code Amphetamine Search Results". U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/scripts/cder/ndc/results.cfm?beginrow=1&numberperpage=160&searchfield=amphetamine&searchtype=ActiveIngredient&OrderBy=ProprietaryName.
- ↑ "Mydayis- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine aspartate monohydrate, and amphetamine sulfate capsule, extended release". 28 October 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=141a7970-3f06-44ea-9ab7-aeece2c085fc.
- ↑ "Adzenys XR-ODT- amphetamine tablet, orally disintegrating". 10 March 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c1179269-00b5-48ea-972d-31e614e99b7e.
- ↑ "Drug Approval Package: Adzenys XR-ODT (amphetamine)". 27 January 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/204326Orig1_toc.cfm.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedEvekeo - ↑ "Evekeo". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=200166.
- ↑ 77.0 77.1 "Vyvanse Prescribing Information". Shire US Inc.. May 2017. pp. 17–21. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021977s045,208510s001lbl.pdf. Retrieved 10 July 2017.
- ↑ "Zenzedi (dextroamphetamine sulfate, USP)". Zenzedi.com. http://zenzedi.com/.
- ↑ "ProCentra (dextroamphetamine sulfate 5 mg/5 mL Oral Solution)". FSC Laboratories. http://www.fsclabs.com/ProCentra.html.
- ↑ Mickle T, Krishnan S, Bishop B, Lauderback C, Moncrief JS, Oberlender R, Piccariello T, Paul BJ, Verbicky CD, "Abuse-resistant amphetamine prodrugs", US patent 7655630, issued 2010, assigned to Takeda Pharmaceutical Co Ltd
- ↑ "Stimulant treatment for attention deficit hyperactivity disorder". Australian Prescriber 18 (3): 60–63. 1995. doi:10.18773/austprescr.1995.064.
- ↑ "Pharmaceutical Services". .health.nsw.gov.au. http://www0.health.nsw.gov.au/PublicHealth/Pharmaceutical/adhd/faqs.asp.
- ↑ "Dexamfetamine sulphate - Medicinal forms". BMJ Group and Pharmaceutical Press (Royal Pharmaceutical Society). https://bnf.nice.org.uk/medicinal-forms/dexamfetamine-sulfate.html.
- ↑ "Dexamfetamine – Prescribe Generically". Red/Amber News (Interface Pharmacist Network Specialist Medicines (IPNSM)) (22): 2. November 2010. http://www.ipnsm.hscni.net/news/RedAmberNewsNov10.pdf. Retrieved 20 April 2012.
- ↑ "Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: a novel d-amphetamine pro-drug". Neuropharmacology 87: 41–50. December 2014. doi:10.1016/j.neuropharm.2014.02.014. PMID 24594478.
- ↑ "NRP-104 (lisdexamphetamine dimesylate)". Pharmacology/Toxicology Review and Evaluation. U.S. Food and Drug Administration. 2006. pp. 18–19. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/021977s000_PharmToxR.pdf.
- ↑ "Advances and considerations in attention-deficit/hyperactivity disorder pharmacotherapy". Acta Medica Iranica 49 (8): 487–498. September 2011. PMID 22009816. http://acta.tums.ac.ir/index.php/acta/article/view/4380. Retrieved 12 March 2014.
- ↑ "A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to D-amfetamine, methylphenidate and modafinil". Neuropharmacology 73: 348–358. October 2013. doi:10.1016/j.neuropharm.2013.05.021. PMID 23748096.
- ↑ "Lisdexamfetamine and immediate release d-amfetamine - differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity". Neuropharmacology 63 (6): 1064–1074. November 2012. doi:10.1016/j.neuropharm.2012.07.008. PMID 22796358.
- ↑ "Molecular Weight Calculator". Lenntech. http://www.lenntech.com/calculators/molecular/molecular-weight-calculator.htm. Retrieved 19 August 2015.
- ↑ 91.0 91.1 "Dextroamphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491568. Retrieved 19 August 2015.
- ↑ 92.0 92.1 "D-amphetamine sulfate". Tocris. 2015. http://www.tocris.com/dispprod.php?ItemId=5305#.VXpspvlViko. Retrieved 19 August 2015.
- ↑ 93.0 93.1 "Amphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491599. Retrieved 19 August 2015.
- ↑ "Dextroamphetamine Saccharate". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491576. Retrieved 19 August 2015.
- ↑ "Amphetamine Aspartate". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491591. Retrieved 19 August 2015.
External links
- "Dextroamphetamine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/dextroamphetamine.
- "PIM 178: Dexamphetamine Sulphate)". Poison Information Monograph. International Programme on Chemical Safety (IPCS) Chemical Safety Information from Intergovernmental organizations (INCHEM). http://www.inchem.org/documents/pims/pharm/pim178.htm.
 |