Chemistry:Perfluorodecanoic acid
From HandWiki
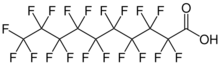
| |
| Names | |
|---|---|
| Preferred IUPAC name
Nonadecafluorodecanoic acid | |
| Other names
PFDA
C10 PFCA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 35659 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10HF19O2 | |
| Molar mass | 514.086 g·mol−1 |
| Melting point | 77–81 °C (171–178 °F; 350–354 K)[1] |
| Boiling point | 218 °C (424 °F; 491 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perfluorodecanoic acid (PFDA) is a fluorosurfactant and has been used in industry,[2] with applications as wetting agent and flame retardant.[3]
It was recently linked to health concerns,[4] like other fluorosurfactants, leading to proposed restrictions on its use.[5] In 2020, a California bill banned its use as an intentionally added ingredient in cosmetics.[6]
It has been proposed as a chemical probe to study peroxisome proliferation.[7][8]
References
- ↑ 1.0 1.1 "Perfluorodecanoic acid 98%". Sigma-Aldrich. https://www.sigmaaldrich.com/catalog/product/aldrich/177741.
- ↑ Reich, Ieva L.; Reich, Hans J.; Menahan, Lawrence A.; Peterson, Richard E. (October 1987). "Synthesis of 14C-labeled perfluorooctanoic and perfluorodecanoic acids; purification of perfluorodecanoic acid". Journal of Labelled Compounds and Radiopharmaceuticals 24 (10): 1235–1244. doi:10.1002/jlcr.2580241011.
- ↑ Harris, M (April 1989). "Developmental toxicity of perfluorodecanoic acid in C57BL/6N mice" (in en). Fundamental and Applied Toxicology 12 (3): 442–448. doi:10.1016/0272-0590(89)90018-3. PMID 2731659.
- ↑ "Danish study links perfluorinated chemicals to miscarriage" (in en). Chemical Watch. https://chemicalwatch.com/23827/danish-study-links-perfluorinated-chemicals-to-miscarriage.
- ↑ "Germany and Sweden propose restrictions on six PFASs" (in en). Chemical Watch. 14 May 2015. https://chemicalwatch.com/62729/germany-and-sweden-propose-restrictions-on-six-pfass.
- ↑ "Assembly Bill No. 2762". State of California. September 30, 2020. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200AB2762.
- ↑ Vanden Heuvel, John P. (October 1996). "Perfluorodecanoic acid as a useful pharmacologic tool for the study of peroxisome proliferation". General Pharmacology: The Vascular System 27 (7): 1123–1129. doi:10.1016/0306-3623(95)00126-3. PMID 8981056.
- ↑ Chen, Li-Chuan; Tatum, Vickie; Glauert, Howard P.; Chow, Ching K. (2001). "Peroxisome proliferator perfluorodecanoic acid alters glutathione and related enzymes" (in en). Journal of Biochemical and Molecular Toxicology 15 (2): 107–113. doi:10.1002/jbt.6. ISSN 1095-6670. PMID 11284052.
 |

