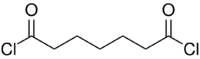
Chemistry:Pimeloyl chloride
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Heptanedioyl dichloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H10Cl2O2 | |
| Molar mass | 197.06 g·mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H315, H318, H335 | |
| P260, P261, P264, P271, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pimeloyl chloride is a di-acyl chloride. It is used as a reagent in organic synthesis.
Synthesis
Pimeloyl chloride can be synthesized from pimelic acid in thionyl chloride.[1]
References
- ↑ "NOVEL TRANSCRIPTION FACTOR MODULATORS" US patent 2014256775
 |