Chemistry:Suberoyl chloride
From HandWiki
| |
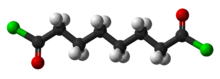
| |
| Names | |
|---|---|
| Preferred IUPAC name
Octanedioyl dichloride | |
| Other names
Suberoyl dichloride; Suberic acid chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H12Cl2O2 | |
| Molar mass | 211.08 g·mol−1 |
| Density | 1.172 g/cm3 |
| Boiling point | 162–163 °C (324–325 °F; 435–436 K) |
| Reacts with water | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| Flash point | 110 °C (230 °F; 383 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
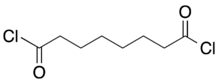
Suberoyl chloride is an organic compound with the formula (CH2)6(COCl)2. It is the diacid chloride derivative of suberic acid. It is a colorless liquid although aged samples appear yellow or even brown.
Uses
Suberoyl chloride is used as a reagent to synthesize hydroxyferrocifen hybrid compounds that have antiproliferative activity against triple negative breast cancer cells. It is also used as a cross-linking agent to cross-link chitosan membranes, and also improves the membrane's integrity.[1]
References
- ↑ "Suberoyl chloride". Alfa Aesar. https://www.alfa.com/en/catalog/B24580/. Retrieved 16 April 2019.
External links
 |