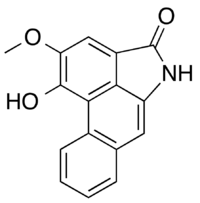
Chemistry:Piperolactam A
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Hydroxy-2-methoxydibenzo[cd,f]indol-4(5H)-one | |
| Other names
Aristolactam FI
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H11NO3 | |
| Molar mass | 265.268 g·mol−1 |
| Melting point | 313 °C (595 °F; 586 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Piperolactam A is a tetracyclic alkaloid found in many plants but first isolated from the roots of Piper longum (long pepper). As a group, such compounds are called aristolactams, and are related to the aristolochic acid found in European birthwort.
History
Piperolactam A was first reported in 1988 after isolation from an extract of Piper longum.[1] Many closely related natural product alkaloids are known including aristolochic acid and its lactam derivatives.[2][3] In some reports, piperolactam A is called aristolactam FI.[4]
Synthesis
Biosynthesis
It has been suggested that piperolactam A and related compounds are biosynthesised from aporphine class precursors.[5]
Chemical synthesis
Aristolactams including piperolactam A have been the subject of total synthesis studies,[4] which have been reviewed.[6]
Natural occurrence
Piperolactam A and related compounds are found in Aristolochiaceae (birthwort), Annonaceae (custard apple), Piperaceae (pepper), and Saururaceae plant families.[2][6]
References
- ↑ Desai, Sanjay J.; Prabhu, Bharathi R.; Mulchandani, Newand B. (1988). "Aristolactams and 4,5-dioxoaporphines from Piper longum". Phytochemistry 27 (5): 1511–1515. doi:10.1016/0031-9422(88)80226-7. Bibcode: 1988PChem..27.1511D.
- ↑ 2.0 2.1 Kumar, Vineet; Poonam; Prasad, Ashok K.; Parmar, Virinder S. (2003). "Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities". Natural Product Reports 20 (6): 565–83. doi:10.1039/B303648K. PMID 14700200.
- ↑ Michl, Johanna; Ingrouille, Martin J.; Simmonds, Monique S. J.; Heinrich, Michael (2014). "Naturally occurring aristolochic acid analogues and their toxicities". Natural Product Reports 31 (5): 676–93. doi:10.1039/c3np70114j. PMID 24691743.
- ↑ 4.0 4.1 Kim, Joa Kyum; Kim, Young Ha; Nam, Ho Tae; Kim, Bum Tae; Heo, Jung-Nyoung (2008). "Total Synthesis of Aristolactams via a One-Pot Suzuki−Miyaura Coupling/Aldol Condensation Cascade Reaction". Organic Letters 10 (16): 3543–3546. doi:10.1021/ol801291k. PMID 18642834.
- ↑ Dos Santos, Anderson R.; Vaz, Nelissa P. (2019). "Isoquinoline Alkaloids and Chemotaxonomy". Biodiversity and Chemotaxonomy. Sustainable Development and Biodiversity. 24. pp. 167–193. doi:10.1007/978-3-030-30746-2_8. ISBN 978-3-030-30745-5.
- ↑ 6.0 6.1 Reddy, Mallu Chenna; Jeganmohan, Masilamani (2017). "Total synthesis of aristolactam alkaloids via synergistic C–H bond activation and dehydro-Diels–Alder reactions". Chemical Science 8 (5): 4130–4135. doi:10.1039/c7sc00161d. PMID 30155216.
 |
