Chemistry:Piprozolin
From HandWiki
Short description: Chemical compound
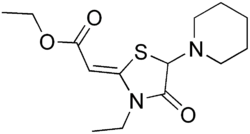 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H22N2O3S |
| Molar mass | 298.40 g·mol−1 |
| |
| (verify) | |
Piprozolin (or piprozoline) is a medication for bile therapy.[1][2]
Synthesis
Compared to fenclozic acid, piprozolin shows choleretic rather than anti-inflammatory activity. That is, the compound is useful in those conditions where the flow of bile is to be increased.
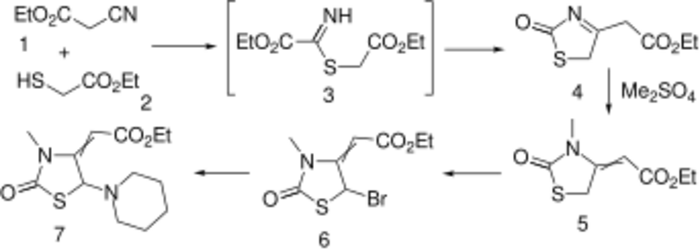
Condensation of ethyl mercaptoacetate with ethyl cyanoacetate leads to thiazolinone (4); an intermediate such as 3, involving addition of the mercaptide to the nitrile function can reasonably be invoked. M/ethylation with di(m)ethyl sulfate proceeds on nitrogen with the concomitant shift of the enamine to afford olefin (5). Bromination of the active methylene (6) followed by displacement of halogen by piperidine affords the choleretic piprozolin (7).
References
- ↑ "[Research with experimental animals on the mechanism of action of the new choleretic, 3-ethyl-4-oxo-5-piperidino-delta 2, alpha-thiazolidine-acetic acid ethyl ester (Piprozolin)]". Archives Internationales de Pharmacodynamie et de Therapie 198 (2): 333–46. August 1972. PMID 5074733.
- ↑ "Piprozolin". Drugs-about.com. http://drugs-about.com/ing/piprozolin.html.
- ↑ "Substituierte 2-Methylen-thiazolidone-(4)". Justus Liebigs Annalen der Chemie 665: 150–165. 1975. doi:10.1002/jlac.19636650118.
- ↑ Satzinger G, Herrmann M, Vollmer K, DE patent 2414345, issued 1975, assigned to Goeecke
- ↑ Satzinger G, Herrmann M, Vollmer K, US patent 3971794, issued 1976, assigned to Warner-Lambert
 |
