Chemistry:Cholic acid

| |
| |
| Names | |
|---|---|
| IUPAC name
3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid
| |
| Systematic IUPAC name
(6R)-6-[(1R,3aS,3bR,4R,5aS,7R,9aS,9bS,11S,11aR)-4,7,11-Trihydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]heptanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H40O5 | |
| Molar mass | 408.57 g/mol |
| Melting point | 200 to 201 °C (392 to 394 °F; 473 to 474 K) |
| −282.3·10−6 cm3/mol | |
| Pharmacology | |
| 1=ATC code }} | A05AA03 (WHO) |
| |
| License data | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
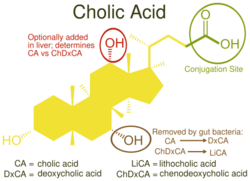
Cholic acid, also known as 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid is a primary bile acid[3] that is insoluble in water (soluble in alcohol and acetic acid), it is a white crystalline substance. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of the two major bile acids produced by the liver, where it is synthesized from cholesterol. These two major bile acids are roughly equal in concentration in humans.[4] Derivatives are made from cholyl-CoA, which exchanges its CoA with either glycine, or taurine, yielding glycocholic and taurocholic acid, respectively.[5]
Cholic acid downregulates cholesterol-7-α-hydroxylase (rate-limiting step in bile acid synthesis), and cholesterol does the opposite. This is why chenodeoxycholic acid, and not cholic acid, can be used to treat gallstones (because decreasing bile acid synthesis would supersaturate the stones even more).[6][7]
Cholic acid and chenodeoxycholic acid are the most important human bile acids. Other species may synthesize different bile acids as their predominant primary bile acids.[8]
Medical uses
Cholic acid, sold under the brand name Cholbam, is approved for use in the United States and is indicated as a treatment for children and adults with bile acid synthesis disorders due to single enzyme defects, and for peroxisomal disorders such as Zellweger syndrome.[9][1][10]
It was approved for use in the European Union in September 2013, and is sold under the brand name Orphacol.[2] It is indicated for the treatment of inborn errors in primary bile-acid synthesis due to 3β-hydroxy-Δ5-C27-steroid oxidoreductase deficiency or Δ4-3-oxosteroid-5β-reductase deficiency in infants, children and adolescents aged one month to 18 years and adults.[2]
Cholic acid FGK (Kolbam) was approved for medical use in the European Union in November 2015.[11] It is indicated for the treatment of inborn errors of primary bile acid synthesis, in infants from one month of age for continuous lifelong treatment through adulthood, encompassing the following single enzyme defects:[11]
- sterol 27-hydroxylase deficiency (presenting as cerebrotendinous xanthomatosis, CTX);[11]
- 2- (or alpha-) methylacyl-CoA racemase (AMACR) deficiency;[11]
- cholesterol 7 alpha-hydroxylase (CYP7A1) deficiency.[11]
The most common side effects include peripheral neuropathy (nerve damage in the hands and feet), diarrhea, nausea (feeling sick), acid reflux (stomach acid flowing up into the mouth), esophagitis (inflammation of the food pipe), jaundice (yellowing of the skin and eyes), skin problems (lesions) and malaise (feeling unwell).[11]
Cancer
A meta-analysis on the relationship of fecal bile acid concentrations to the development and progression of colorectal cancer was reported in 2025.[12] It was found that higher fecal concentrations of cholic acid are associated with a higher risk/incidence of colorectal cancer.[12] Bile acids have been implicated as carcinogens in the gastrointestinal tract.[13]
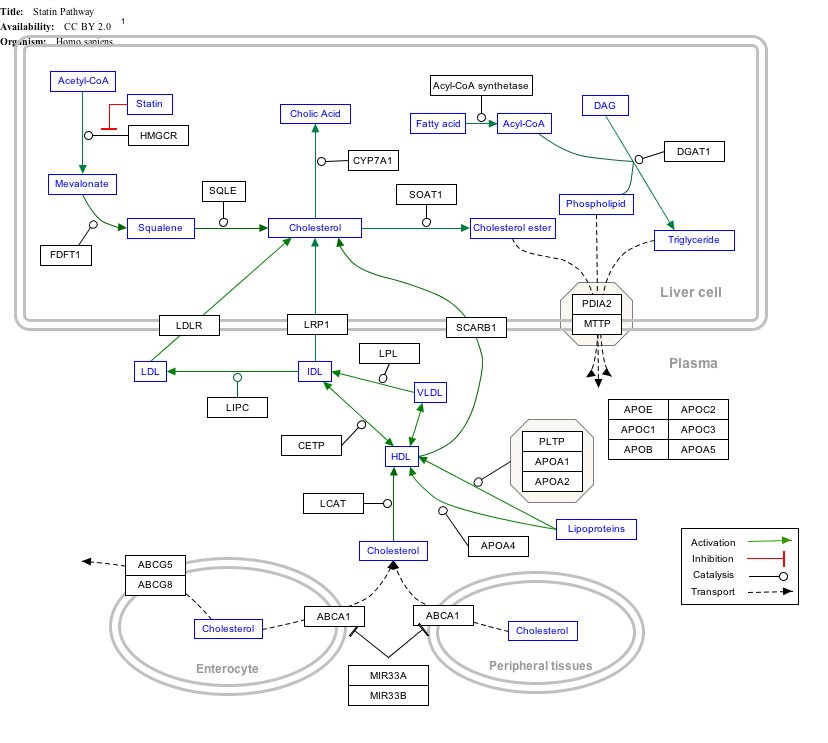
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ↑ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430". http://www.wikipathways.org/index.php/Pathway:WP430.
References
- ↑ 1.0 1.1 "Cholbam- cholic acid capsule". 12 July 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b67402-8550-4604-97a0-c7b149fbf753.
- ↑ 2.0 2.1 2.2 "Orphacol EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/orphacol. Text was copied from this source which is under copyright of the European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Colleen Smith; Lieberman, Michael; Marks, Dawn B.; Allan D. Marks (2007). Marks' essential medical biochemistry. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-9340-7.
- ↑ "Effects of oral contraceptives on the gallbladder bile of normal women". N. Engl. J. Med. 294 (4): 189–92. January 1976. doi:10.1056/NEJM197601222940403. PMID 1244533.
- ↑ Chiang JY (October 2009). "Bile acids: regulation of synthesis". Journal of Lipid Research 50 (10): 1955–66. doi:10.1194/jlr.R900010-JLR200. PMID 19346330.
- ↑ "Chenodeoxycholic acid treatment of gallstones. A follow-up report and analysis of factors influencing response to therapy". The New England Journal of Medicine 293 (8): 378–83. August 1975. doi:10.1056/NEJM197508212930804. PMID 1152936.
- ↑ Alan F. Hofmann; Johnson L. Thistle; Peter D. Klein; Patricia A. Szczepanik; Paulina Y. S. Yu (1978). "Chenotherapy for Gallstone Dissolution, II. Induced Changes in Bile Composition and Gallstone Response". JAMA 239 (12): 1138–1144. doi:10.1001/jama.1978.03280390034017. PMID 628065.
- ↑ "Bile salts of vertebrates: structural variation and possible evolutionary significance". J. Lipid Res. 51 (2): 226–46. February 2010. doi:10.1194/jlr.R000042. PMID 19638645.
- ↑ "Cholbam (cholic acid) Capsules". 13 April 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205750Orig1s000TOC.cfm.
- ↑ "FDA approves Cholbam to treat rare bile acid synthesis disorders". U.S. Food and Drug Administration (FDA) (Press release). 17 March 2015. Archived from the original on 26 January 2018. Retrieved 19 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "Kolbam EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/kolbam. Text was copied from this source which is under copyright of the European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 12.0 12.1 "The effect of fecal bile acids on the incidence and risk-stratification of colorectal cancer: an updated systematic review and meta-analysis". Sci Rep 15 (1). January 2025. doi:10.1038/s41598-024-84801-6. PMID 39753873. Bibcode: 2025NatSR..15..740Y.
- ↑ "Bile acids as carcinogens in the colon and at other sites in the gastrointestinal system". Exp Biol Med (Maywood) 248 (1): 79–89. January 2023. doi:10.1177/15353702221131858. PMID 36408538.
 |