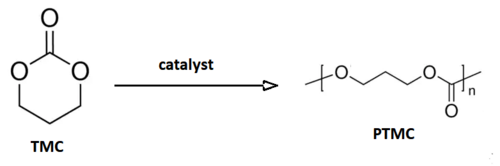
Chemistry:Poly(trimethylene carbonate)
| |
| Names | |
|---|---|
| IUPAC name
poly(trimethylene carbonate), poly(1,3-Dioxan-2-one)
| |
| Other names
Poly(TMC), 1,3-Dioxan-2-one homopolymer
| |
| Identifiers | |
| Properties | |
| (C4H6O3)n | |
| Melting point | 38 to 41°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases.[citation needed] Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.[1]
Synthesis
In opposition to five-membered cyclic carbonate, the six-membered ones like trimethylene carbonate are thermodynamically less stable than its polymer, undergoing the ring-opening polymerization with retention of CO[math]\displaystyle{ _2 }[/math] in the polymer structure, and generation an aliphatic polycarbonate.[1]
Ring-opening polymerization (ROP) is the most common method used to synthesize poly(trimethylene carbonate) and their copolymers, since this synthetic route can allow mild reaction condition.[citation needed]
Several ROP catalysts/initiators have been used to synthesize the polymer, among them metal-catalyzed polymerization using oxides, salts and complexes of Al, K, Ti, Zn, Zr, Sn and rare earths metals; enzyme-catalyzed polymerization; and alcohol-initiated polymerization.[2][3]
Physical properties
PTMC is a predominantly amorphous polymer in the relaxed state but it can present some crystallinity, particularly when the chains are stretched.[4]
The polymer presents glass transition temperature ([math]\displaystyle{ T_g }[/math]) between -15 and -30 °C and melting temperature ([math]\displaystyle{ T_m }[/math]) ranging from 38 to 41°C.[5]
Low molecular weight PTMC is a rubbery polymer with poor dimensional stability, tackiness, and inadequate mechanical properties. Nevertheless, high molecular weight amorphous PTMC (over 100,000) is very flexible, with a relatively low elastic modulus (5–7 MPa) at room temperature, tough and it presents excellent ultimate mechanical properties. Mechanical properties of the rubber can be also improved upon cross-linking by gamma-irradiation.[4][6]
PTMC has resistance to non-enzymatic hydrolysis, compared to most aliphatic polyesters, but it is biodegradable in vivo by enzymes. It is a resorbable material since the ester bonds can be enzymatically broken, producing CO[math]\displaystyle{ _2 }[/math] and water. So, in vivo, it degrades by surface erosion and its decomposition products contain no organic acids, preventing potential inflammatory responses.[7]
Applications
Due to the predominant amorphous nature, PTMC is a flexible polymer with rubbery behavior. In addition, the biodegradability and biocompatibility of PTMC make it to have high applicability in biomedical applications as scaffolds for tissue regeneration and drug delivery devices.
PTMC has been used as scaffolds for tissue engineering particularly for some types of soft tissues in which the maintenance of mechanical properties is important for tissue reconstruction. PTMC-based membranes have been also evaluated as barrier for use in hard tissue guided regeneration like bone. The performance of these membranes is comparable with commercial collagen and e-PTFE membranes, showing well suitability for use in guided bone regeneration.[4] [6]
Due to rubbery and hydrophobic nature, PTMC-based copolymers produced from ROP of TMC with lactone-based comomers have been synthesized to modify these characteristics, amplifying applications. Thus, the use as resorbable medical devices in which control of rigidity and biodegradation time are desired has been proposed. Main examples of these copolymers are poly(L-lactide-co-trimethylene carbonate),[8] poly(glycolide-co-trimethylene carbonate) [9] and poly(caprolactone-co-trimethylene carbonate).[10]
Poly(L-lactide-co-trimethylene carbonate) has been proposed for application as small diameter vascular grafts.[8] Poly(glycolide-co-trimethylene carbonate) is a commercial monofilament used for suture with slow biodegradation rate which allows maintenance of high mechanical strength compatible with the surgical recovery.[9] Poly(caprolactone-co-trimethylene carbonate) has been proposed as biomaterial for conduits in the regeneration of central nervous system.[10]
References
- ↑ 1.0 1.1 Darensbourg, Donald J.; Horn, Adolfo Jr.; Moncada, Adriana I. (Aug 2010). "A facile catalytic synthesis of trimethylene carbonate from trimethylene oxide and carbon dioxide" (in en). Green Chemistry 12 (8): 1376–1379. doi:10.1039/C0GC00136H. ISSN 1463-9270. https://pubs.rsc.org/en/content/articlelanding/2010/gc/c0gc00136h.
- ↑ Okada, Takao; Imamura, Yukari; Matsuda, Takehisa (2010-04-01). "Polymerization of trimethylene carbonate in aqueous solutions: Reaction mechanism and characterization: Polymerization of Trimethylene Carbonate" (in en). Journal of Polymer Science Part A: Polymer Chemistry 48 (7): 1485–1492. doi:10.1002/pola.23891. https://onlinelibrary.wiley.com/doi/10.1002/pola.23891.
- ↑ Brignou, Pierre; Guillaume, Sophie M.; Roisnel, Thierry; Bourissou, Didier; Carpentier, Jean-François (Jul 2012). "Discrete Cationic Zinc and Magnesium Complexes for Dual Organic/Organometallic-Catalyzed Ring-Opening Polymerization of Trimethylene Carbonate" (in en). Chemistry – A European Journal 18 (30): 9360–9370. doi:10.1002/chem.201200336. PMID 22736527. https://onlinelibrary.wiley.com/doi/10.1002/chem.201200336.
- ↑ 4.0 4.1 4.2 Pêgo, Ana Paula; Grijpma, Dirk W.; Feijen, Jan (Oct 2003). "Enhanced mechanical properties of 1,3-trimethylene carbonate polymers and networks" (in en). Polymer 44 (21): 6495–6504. doi:10.1016/S0032-3861(03)00668-2. https://linkinghub.elsevier.com/retrieve/pii/S0032386103006682.
- ↑ Brossier, Thomas; Volpi, Gael; Lapinte, Vincent; Blanquer, Sebastien (Jan 2021). "Synthesis of Poly(Trimethylene Carbonate) from Amine Group Initiation: Role of Urethane Bonds in the Crystallinity" (in en). Polymers 13 (2): 280. doi:10.3390/polym13020280. ISSN 2073-4360. PMID 33467051.
- ↑ 6.0 6.1 van Leeuwen, A. C.; Huddleston Slater, J. J. R.; Gielkens, P. F. M.; de Jong, J. R.; Grijpma, D. W.; Bos, R. R. M. (2012-04-01). "Guided bone regeneration in rat mandibular defects using resorbable poly(trimethylene carbonate) barrier membranes" (in en). Acta Biomaterialia 8 (4): 1422–1429. doi:10.1016/j.actbio.2011.12.004. ISSN 1742-7061. PMID 22186161. https://www.sciencedirect.com/science/article/pii/S174270611100540X.
- ↑ Mindemark, Jonas; Hilborn, Jöns; Bowden, Tim (Apr 2007). "End-Group-Catalyzed Ring-Opening Polymerization of Trimethylene Carbonate" (in en). Macromolecules 40 (10): 3515–3517. doi:10.1021/ma0629081. ISSN 0024-9297. Bibcode: 2007MaMol..40.3515M. https://pubs.acs.org/doi/10.1021/ma0629081.
- ↑ 8.0 8.1 Braghirolli, D.I.; Caberlon, B.; Gamba, D.; Petry, Jftc.; Dias, M.L.; Pranke, P. (2019). "Poly(trimethylene carbonate-co-L-lactide) electrospun scaffolds for use as vascular grafts". Brazilian Journal of Medical and Biological Research 52 (8): e8318. doi:10.1590/1414-431x20198318. ISSN 1414-431X. PMID 31411247.
- ↑ 9.0 9.1 Noorsal, K.; Mantle, M. D.; Gladden, L. F.; Cameron, R. E. (May 2005). "Degradation and drug-release studies of a poly(glycolide-co-trimethylene carbonate) copolymer (Maxon)" (in en). Journal of Applied Polymer Science 95 (3): 475–486. doi:10.1002/app.21108. ISSN 0021-8995. https://onlinelibrary.wiley.com/doi/10.1002/app.21108.
- ↑ 10.0 10.1 Rocha, Daniela Nogueira; Brites, Pedro; Fonseca, Carlos; Pêgo, Ana Paula (Feb 2014). "Poly(Trimethylene Carbonate-co-ε-Caprolactone) Promotes Axonal Growth" (in en). PLOS ONE 9 (2): e88593. doi:10.1371/journal.pone.0088593. ISSN 1932-6203. PMID 24586346. Bibcode: 2014PLoSO...988593R.
 |