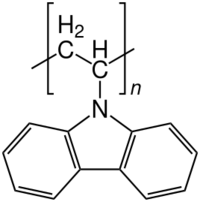
Chemistry:Polyvinylcarbazole
| |
| Names | |
|---|---|
| Other names
Poly(vinylcarbazole); Poly(N-vinylcarbazole); Poly(9-vinylcarbazole)
| |
| Identifiers | |
| Properties | |
| (C14H11N)n | |
| Melting point | > 320 °C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polyvinylcarbazole (PVK) is a temperature-resistant[2] thermoplastic polymer produced by radical polymerization from the monomer N-vinylcarbazole. It is a photoconductive polymer and thus the basis for photorefractive polymers and organic light-emitting diodes.[3]
History
Polyvinylcarbazole was discovered by the chemists Walter Reppe (1892-1969), Ernst Keyssner and Eugen Dorrer and patented by I.G. Farben in the USA in 1937.[4][1] PVC was the first polymer whose photoconductivity was known. Starting in the 1960s, further polymers of this kind were sought.[3]
Production
Polyvinylcarbazole is obtained from N-vinylcarbazole by radical polymerization in various ways. It can be produced by suspension polymerization at 180 °C with sodium chloride and potassium chromate as catalyst.[2] Alternatively, AIBN can also be used as a radical starter or a Ziegler-Natta catalyst.[1]
Properties
Physical properties
PVK can be used at temperatures of up to 160 - 170 °C and is therefore a temperature-resistant thermoplastic. The electrical conductivity changes depending on the illumination. For this reason, PVK is classified as a semiconductor or photoconductor. The polymer is extremely brittle, but the brittleness can be reduced by copolymerization with a little isoprene.[5]
Chemical properties
Polyvinylcarbazole is soluble in aromatic hydrocarbons, halogenated hydrocarbons and ketones.[1] It is resistant to acids, alkalis, polar solvents and aliphatic hydrocarbons.[2] The addition of PVC to other plastic masses increases their temperature resistance.
Use
Due to its high price and special properties, the use of PVK is limited to special areas.[2] It is used in insulation technology,[2] electrophotography (e.g. in copiers and laser printers),[3] for the fabrication of polymer photonic crystals,[6] for organic light-emitting diodes and photovoltaic devices.[1] In addition, PVC is a well researched component in photorefractive polymers and therefore plays an important role in holography. Another application is the production of cooking-proof copolymers with styrene.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Wypych, George (2016) (in German). Handbook of Polymers. Elsevier. pp. 643–645. ISBN 978-1-927885-11-6. https://books.google.com/books?id=aedxCQAAQBAJ&pg=PA269.
- ↑ 2.0 2.1 2.2 2.3 2.4 Hans-Dieter Jakubke, Ruth Karcher (Koordinatoren): Lexikon der Chemie in drei Bänden, Spektrum Verlag, Band 3, Heidelberg 1999, ISBN:3-8274-0381-2, S. 92.
- ↑ 3.0 3.1 3.2 Tsutsumi, Naoto (2016). "Molecular design of photorefractive polymers". Polymer Journal 48 (5): 571–588. doi:10.1038/pj.2015.131..
- ↑ US-Patent Nr. 2,072,465 pdfpiw.uspto.gov: Production of polymeric N-Vinyl compounds. Priorität 24. Juli 1934, veröffentlicht am 2. März 1937, Anmelder: I.G. Farbenindustrie Aktiengesellschaft, Erfinder: Walter Reppe, Ernst Keyssner, Eugen Dorrer.
- ↑ Elias, Hans-Georg (2001). "Poly(N-vinylcarbazol)". Makromoleküle (Industrielle Polymere und Synthesen Volume 3). Weinheim: Wiley-VCH. p. 211. ISBN 3-527-29961-0. https://books.google.com/books?id=F6xau0tvOUgC&pg=PA211.).
- ↑ Lova, Paola; Megahd, Heba; Stagnaro, Paola; Alloisio, Marina; Patrini, Maddalena; Comoretto, Davide (2020-06-15). "Strategies for Dielectric Contrast Enhancement in 1D Planar Polymeric Photonic Crystals" (in en). Applied Sciences 10 (12): 4122. doi:10.3390/app10124122. ISSN 2076-3417.
 |


