Chemistry:Potassium phthalimide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H4KNO2 | |
| Molar mass | 185.221 g/mol |
| Appearance | Light yellow solid |
| Melting point | > 300 °C (572 °F; 573 K) |
| Soluble in water | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Phthalimide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
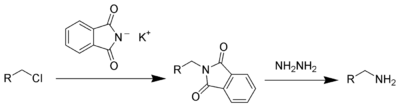
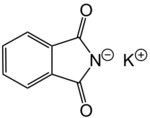
Potassium phthalimide is a chemical compound of formula C8H4KNO2. It is the potassium salt of phthalimide, and usually presents as fluffy, very pale yellow crystals. It can be prepared by adding a hot solution of phthalimide in ethanol to a solution of potassium hydroxide in ethanol; the desired product precipitates.[1]
This compound is a commercially available reagent used in the Gabriel synthesis of amines.
References
- ↑ P. L. Salzberg and J. V. Supniewski (1941). "β-Bromoethylphthalimide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0119.; Collective Volume, 1, pp. 119
 |