Chemistry:Potassium thioacetate
From HandWiki
Short description: Organosulfur compound (CH3COS- K+)
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | C005732 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H3KOS | |
| Molar mass | 114.21 |
| Appearance | white solid |
| good | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
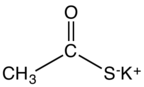
Potassium thioacetate is an organosulfur compound and a salt with the formula CH
3COS−
K+
. This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives.[1]
Synthesis and reactions
Potassium thioacetate, which is commercially available, can be prepared by combining acetyl chloride and potassium hydrogen sulfide:
- [math]\ce{ CH3COCl + 2 KSH -> KCl + CH3COSK + H2S }[/math]
It arises also by the neutralization of thioacetic acid with potassium hydroxide.
Use in preparation of thiols
In a common application, potassium thioacetate is combined with alkylating agents to give thioacetate esters (X = halide):
- [math]\ce{ CH3COSK + RX -> CH3COSR + KX }[/math]
Hydrolysis of these esters affords thiols:
- [math]\ce{ CH3COSR + H2O -> CH3CO2H + RSH }[/math]
The thioacetate esters can also be cleaved with methanethiol in the presence of stoichiometric base, as illustrated in the preparation of pent-4-yne-1-thiol:[2]
- [math]\ce{ H3C(CH2)3OMs + KSAc -> H3C(CH2)3SAc + KOMs }[/math]
- [math]\ce{ H3C(CH2)3SAc + HSMe -> H3C(CH2)3SH + MeSAc }[/math]
References
- ↑ Zongjun Qiao and Xuefeng Jiang "Potassium Thioacetate" e-EROS Encyclopedia Of Reagents For Organic Synthesis, 2014. doi:10.1002/047084289X.rn01737
- ↑ Matteo Minozzi; Daniele Nanni; Piero Spagnolo (2008). "4-Pentyne-1-thiol". EEROS. doi:10.1002/047084289X.rn00855. ISBN 978-0-471-93623-7.
 |

