Chemistry:Pseudoginsenoside F11
| |
| Names | |
|---|---|
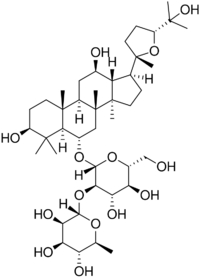
| IUPAC name
(24R)-3β,12β,25-Trihydroxy-20,24-epoxydammaran-6α-yl α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside
| |
| Systematic IUPAC name
(2S,3R,4R,5R,6S)-2-{[(2R,3R,4S,5S,6R)-1-({(1S,3aR,3bR,5S,5aR,7S,9aR,9bR,11R,11aR)-7,11-Dihydroxy-1-[(2S,5R)-5-(2-hydroxypropan-2-yl)-2-methyloxolan-2-yl]-3a,3b,6,6,9a-pentamethylhexadecahydro-5H-cyclopenta[a]phenanthren-5-yl}oxy)-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy}-6-methyloxane-3,4,5-triol | |
| Other names
Ginsenoside A1
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| C42H72O14 | |
| Molar mass | 801.024 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pseudoginsenoside F11 is a chemical natural product found in American ginseng (Panax quinquefolius) but not in Asian ginseng (Panax ginseng), although it has similar properties to the Asian ginseng compound ginsenoside Rf.[1] The molecule is a triterpenoid saponin member of the dammarane family and contains a four-ring rigid skeleton.[1] Compounds in the ginsenoside family are found almost exclusively in plants of the genus Panax. A wide variety of difficult-to-characterize in vitro effects have been reported for the compounds in isolation.[2][3] Pseudoginsenoside F11 and its derivatives are sometimes referred to as having an ocotillol-type skeleton structure.[1][4]
Studies in mice have identified antagonistic effects on the actions of other well-characterized drugs, such as scopolamine,[5] morphine,[6][7] and methamphetamine.[8]
References
- ↑ 1.0 1.1 1.2 Qi, LW; Wang, CZ; Yuan, CS (June 2011). "Ginsenosides from American ginseng: chemical and pharmacological diversity.". Phytochemistry 72 (8): 689–99. doi:10.1016/j.phytochem.2011.02.012. PMID 21396670.
- ↑ Attele, AS; Wu, JA; Yuan, CS (1 December 1999). "Ginseng pharmacology: multiple constituents and multiple actions.". Biochemical Pharmacology 58 (11): 1685–93. doi:10.1016/s0006-2952(99)00212-9. PMID 10571242.
- ↑ Christensen, LP (2009). "Ginsenosides chemistry, biosynthesis, analysis, and potential health effects.". Advances in Food and Nutrition Research 55: 1–99. doi:10.1016/S1043-4526(08)00401-4. PMID 18772102.
- ↑ Fuzzati, N (5 December 2004). "Analysis methods of ginsenosides.". Journal of Chromatography B 812 (1–2): 119–33. doi:10.1016/j.jchromb.2004.07.039. PMID 15556492.
- ↑ Li, Z; Guo, YY; Wu, CF; Li, X; Wang, JH (April 1999). "Protective effects of pseudoginsenoside-F11 on scopolamine-induced memory impairment in mice and rats.". The Journal of Pharmacy and Pharmacology 51 (4): 435–40. doi:10.1211/0022357991772484. PMID 10385216.
- ↑ Li, Z; Wu, CF; Pei, G; Guo, YY; Li, X (July 2000). "Antagonistic effect of pseudoginsenoside-F11 on the behavioral actions of morphine in mice.". Pharmacology Biochemistry and Behavior 66 (3): 595–601. doi:10.1016/s0091-3057(00)00260-4. PMID 10899376.
- ↑ Hao, Y; Yang, JY; Wu, CF; Wu, MF (April 2007). "Pseudoginsenoside-F11 decreases morphine-induced behavioral sensitization and extracellular glutamate levels in the medial prefrontal cortex in mice.". Pharmacology Biochemistry and Behavior 86 (4): 660–6. doi:10.1016/j.pbb.2007.02.011. PMID 17368734.
- ↑ Wu, CF; Liu, YL; Song, M; Liu, W; Wang, JH; Li, X; Yang, JY (August 2003). "Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice.". Pharmacology Biochemistry and Behavior 76 (1): 103–9. doi:10.1016/s0091-3057(03)00215-6. PMID 13679222.
 |

