Chemistry:Pyridine-3-carbaldehyde
From HandWiki
Short description: Organic compound
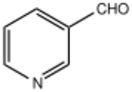
| |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridine-3-carbaldehyde | |
| Other names
Nicotinaldehyde, 3-formylpyridine, 3-pyridinaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H5NO | |
| Molar mass | 107.112 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.14 g/cm3 |
| Melting point | 7 °C (45 °F; 280 K) |
| Boiling point | 95–97 °C (203–207 °F; 368–370 K) 15 mm |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H226, H302, H315, H317, H318, H334, H335, H341, H412 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P272, P273, P280, P281, P285, P301+312, P302+352, P303+361+353, P304+340, P304+341, P305+351+338, P308+313, P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Pyridine-3-carbaldehyde is an organic compound with the formula C5H4NCHO. It is one of three isomeric pyridinaldehydes. The other isomers are pyridine-2-carboxaldehyde and pyridine-4-carboxaldehyde. It is a colorless liquid that is routinely available commercially. It can be produced from nicotinonitrile. Alternatively, it arises by the aerobic oxidation of the corresponding alcohol.[1]
Safety
3-Pyridinecarboxaldehyde is a severe skin irritant.[2]
References
- ↑ Marko, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. (1996). "Copper-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones: An Efficient, Aerobic Alternative". Science 274 (5295): 2044–2046. doi:10.1126/science.274.5295.2044. PMID 8953027.
- ↑ H. Stetter1, H. Kuhlmann, and G. Lorenz (1979). "Cyanide-Catalyzed Conjugate Addition of Aryl Aldehydes: 4-Oxo-4-(3-Pyridyl)Butyronitrile". Organic Syntheses 59: 53. doi:10.15227/orgsyn.059.0053.

