Chemistry:Rhenium trioxide fluoride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| FO3Re | |
| Molar mass | 253.202 g·mol−1 |
| Appearance | white |
| Density | 6.042 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhenium trioxide fluoride is an inorganic compound with the formula ReO
3F. It is a white, sublimable, diamagnetic solid, although impure samples appear colored. It one of the few oxyfluorides of rheniium, the other major one being rhenium dioxide trifluoride ReO
2F
3 . The material has no applications, but it is of some academic interest as a rare example of a trioxide fluoride.
Synthesis and reactions
Rhenium trioxide fluoride can be prepared by fluorination of rhenium trioxide:[1]
- 2 ReO
3 + F
2 → 2ReO
3F
With Lewis bases (L) the compound forms adducts with the formula ReO
3FL
2, e.g., L = diethyl ether and acetonitrile.[1]
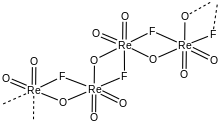
According to X-ray crystallography, the compound adopts a helical chain structure featuring octahedral Re centers linked by two fluoride and two oxide bridging ligands. In contrast with ReO
3F, TcO
3F and MnO
3F crystallize with simpler structures. The Mn compound crystallizes as a tetrahedral monomer. The technetium compound TcO
3F crystallizes as dimers with fluoride bridges.[2] Also contrasting with the structure of rhenium trioxide fluoride is that of rhenium trioxide chloride, which is a monomer.
References
- ↑ 1.0 1.1 Supeł, Joanna; Marx, Rupert; Seppelt, Konrad (2005). "Preparation and Structure of Rhenium Fluoride Trioxide ReO3F, and the Polymorphism of Rhenium Trifluoride Dioxide, ReO2F3". Zeitschrift für anorganische und allgemeine Chemie 631 (15): 2979–2986. doi:10.1002/zaac.200500239.
- ↑ Supeł, Joanna; Abram, Ulrich; Hagenbach, Adelheid; Seppelt, Konrad (2007). "Technetium Fluoride Trioxide, TcO3F, Preparation and Properties". Inorganic Chemistry 46 (14): 5591–5595. doi:10.1021/ic070333y. PMID 17547395.
 |

