Chemistry:Rhenium dioxide trifluoride
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| F3O2Re | |
| Molar mass | 275.200 g·mol−1 |
| Appearance | white |
| Density | 5.161 g/cm3 |
| Melting point | 35 °C; 95 °F; 308 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhenium dioxide trfluoride is an inorganic compound with the formula ReO
2F
3. A white diamagnetic solid, it one of the few oxyfluorides of rhenium, another being rhenium trioxide fluoride, ReO
3F. The material is of some academic interest as a rare example of an dioxide trifluoride.[1] It can be prepared by the reaction of xenon difluoride and rhenium trioxide chloride:
- 2 ReO
3Cl + 3 XeF
2 → 2 ReO
2F
3 + O
2 + Cl
2 + 3 Xe
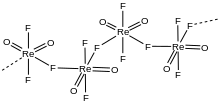
According to X-ray crystallography, the compound can exist in four polymorphs. Two polymorphs adopt chain-like structures featuring octahedral Re centers linked by [bridging bridging fluoride]]s. Two other polymorphs adopt cyclic structures (ReO
2F
3)
3 and (ReO
2F
3)
4, again featuring octahedral Re centers and bridging fluorides. Like related oxyfluorides, these coordination oligomers break up in the presence of Lewis bases. Adducts of the formula ReO
2F
3L where L = acetonitrile have been crystallized.[2]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Supeł, Joanna; Marx, Rupert; Seppelt, Konrad (2005). "Preparation and Structure of Rhenium Fluoride Trioxide ReO3F, and the Polymorphism of Rhenium Trifluoride Dioxide, ReO2F3". Zeitschrift für anorganische und allgemeine Chemie 631 (15): 2979–2986. doi:10.1002/zaac.200500239.
 |

