Chemistry:S-Methylcysteine
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
S-Methyl-L-cysteine
| |
| Systematic IUPAC name
2-amino-3-(methylthio)propanoic acid | |
| Other names
3-methylthioalanine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C4H9NO2S | |
| Molar mass | 135.18 g·mol−1 |
| Appearance | white solid |
| Melting point | 248 °C (478 °F; 521 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
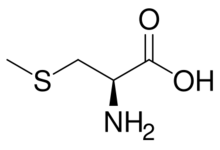
S-Methylcysteine is the amino acid with the nominal formula CH3SCH2CH(NH2)CO2H. It is the S-methylated derivative of cysteine. This amino acid occurs widely in plants, including many edible vegetables.[1]
Biosynthesis
The amino acid is not genetically coded, but it arises by post-translational methylation of cysteine. One pathway involves methyl transfer from alkylated DNA by zinc-cysteinate-containing repair enzymes.[2][3]
Beyond its biological context, it has been examined as a chelating agent.[4]
References
- ↑ Maw, George A. (1982). "Biochemistry of S-Methyl-L-Cysteine and its Principal Derivatives". Sulfur Reports 2: 1–26. doi:10.1080/01961778208082422.
- ↑ Sors, Thomas G.; Ellis, Danielle R.; Na, Gun Nam; Lahner, Brett; Lee, Sangman; Leustek, Thomas; Pickering, Ingrid J.; Salt, David E. (2005). "Analysis of Sulfur and Selenium Assimilation in Astragalus plants with Varying Capacities to Accumulate Selenium". The Plant Journal 42 (6): 785–797. doi:10.1111/j.1365-313X.2005.02413.x. PMID 15941393.
- ↑ Clarke, Steven G. (2018). "The ribosome: A Hot Spot for the Identification of New Types of Protein Methyltransferases". Journal of Biological Chemistry 293 (27): 10438–10446. doi:10.1074/jbc.AW118.003235. PMID 29743234.
- ↑ He, Haiyang; Lipowska, Malgorzata; Xu, Xiaolong; Taylor, Andrew T.; Carlone, Maria; Marzilli, Luigi G. (2005). "Re(CO)3 Complexes Synthesized via an Improved Preparation of Aqueousfac-[Re(CO)3(H2O)3]+as an Aid in Assessing 99m Tc Imaging Agents. Structural Characterization and Solution Behavior of Complexes with Thioether-Bearing Amino Acids as Tridentate Ligands". Inorganic Chemistry 44 (15): 5437–5446. doi:10.1021/ic0501869. PMID 16022542.
 |

