Chemistry:Schotten–Baumann reaction
| Schotten-Baumann reaction | |
|---|---|
| Named after | Carl Schotten Eugen Baumann |
| Reaction type | Condensation reaction |
| Identifiers | |
| Organic Chemistry Portal | schotten-baumann-reaction |
| RSC ontology ID | RXNO:0000165 |
The Schotten–Baumann reaction is a method to synthesize amides from amines and acid chlorides:
Schotten–Baumann reaction also refers to the conversion of acid chloride to esters. The reaction was first described in 1883 by Germany chemists Carl Schotten and Eugen Baumann.[1][2]
The name "Schotten–Baumann reaction conditions" often indicate the use of a two-phase solvent system, consisting of water and an organic solvent. The base within the water phase neutralizes the acid, generated in the reaction, while the starting materials and product remain in the organic phase, often dichloromethane or diethyl ether.
Applications
The Schotten–Baumann reaction or reaction conditions are widely used in organic chemistry.[3][4][5]
Examples:
- synthesis of N-vanillyl nonanamide, also known as synthetic capsaicin
- synthesis of benzamide from benzoyl chloride and a phenethylamine
- synthesis of flutamide, a nonsteroidal antiandrogen
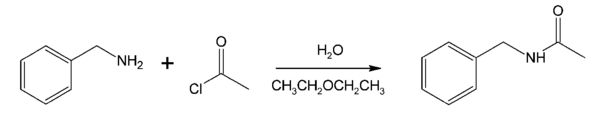
- acylation of a benzylamine with acetyl chloride (acetic anhydride is an alternative)
in the Fischer peptide synthesis (Emil Fischer, 1903)[6] an α-chloro acid chloride is condensed with the ester of an amino acid. The ester is then hydrolyzed and the acid converted to the acid chloride enabling the extension of the peptide chain by another unit. In a final step the chloride atom is replaced by an amino group completing the peptide synthesis.
Further reading
- Schotten, C. (1884). "Ueber die Oxydation des Piperidins". Berichte der deutschen chemischen Gesellschaft 17 (2): 2544–2547. doi:10.1002/cber.188401702178. https://zenodo.org/record/1425345.
- Baumann, E. (1886). "Ueber eine einfache Methode der Darstellung von Benzoësäureäthern". Berichte der deutschen chemischen Gesellschaft 19 (2): 3218–3222. doi:10.1002/cber.188601902348. https://zenodo.org/record/1425451.
See also
References
- ↑ W Pötsch. Lexikon bedeutender Chemiker (VEB Bibliographisches Institut Leipzig, 1989) (ISBN:3-323-00185-0)
- ↑ M B Smith, J March. March's Advanced Organic Chemistry (Wiley, 2001) (ISBN:0-471-58589-0)
- ↑ Kent, R. E.; McElvain, S. M. (1945). "Isobutyramide". Organic Syntheses 25: 58. doi:10.15227/orgsyn.025.0058.
- ↑ Cope, Arthur C.; Ciganek, Engelbert (1959). "N,N-Dimethylcyclohexylmethylamine". Organic Syntheses 39: 19. doi:10.15227/orgsyn.039.0019.
- ↑ X. Wang; S. O. de Silva; J. N. Reed; R. Billadeau; E. J. Griffen; A. Chan; V. Snieckus (1995). "7-Methoxyphthalide". Organic Syntheses 72: 163. doi:10.15227/orgsyn.072.0163.
- ↑ Emil Fischer (1903). "Synthese von Polypeptiden". Berichte der deutschen chemischen Gesellschaft 36 (3): 2982–2992. doi:10.1002/cber.19030360356. https://zenodo.org/record/1426082.
 |

