Chemistry:Lactucopicrin
From HandWiki
| |
| Names | |
|---|---|
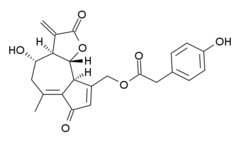
| Preferred IUPAC name
[(3aR,4S,9aS,9bR)-4-Hydroxy-6-methyl-3-methylidene-2,7-dioxo-2,3,3a,4,5,7,9a,9b-octahydroazuleno[4,5-b]furan-9-yl]methyl (4-hydroxyphenyl)acetate | |
| Other names
Intybin
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| MeSH | Intybin |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C23H22O7 | |
| Molar mass | 410.422 g·mol−1 |
| Pharmacology | |
| Oral, Smoked | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lactucopicrin (Intybin) is a bitter substance that has a sedative and analgesic effect,[1] acting on the central nervous system. It is a sesquiterpene lactone, and is a component of lactucarium, derived from the plant Lactuca virosa (wild lettuce), as well as being found in some related plants such as Cichorium intybus.[2] It is also found in dandelion coffee.
As well as their traditional use as sedatives and analgesics, these plants have also been used as antimalarials, and both lactucin and lactucopicrin have demonstrated antimalarial effects in vitro.[3] Lactucopicrin has also been shown to act as an acetylcholinesterase inhibitor.[4]
See also
References
- ↑ Wesołowska, A; Nikiforuk, A; Michalska, K; Kisiel, W; Chojnacka-Wójcik, E (Sep 2006). "Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice". Journal of Ethnopharmacology 107 (2): 254–8. doi:10.1016/j.jep.2006.03.003. PMID 16621374.
- ↑ Sessa, RA; Bennett, MH; Lewis, MJ; Mansfield, JW; Beale, MH (Sep 2000). "Metabolite profiling of sesquiterpene lactones from Lactuca species. Major latex components are novel oxalate and sulfate conjugates of lactucin and its derivatives". Journal of Biological Chemistry 275 (35): 26877–84. doi:10.1074/jbc.M000244200. PMID 10858433.
- ↑ Bischoff, TA; Kelley, CJ; Karchesy, Y; Laurantos, M; Nguyen-Dinh, P; Arefi, AG (2004). "Antimalarial activity of lactucin and lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L.". Journal of Ethnopharmacology 95 (2–3): 455–7. doi:10.1016/j.jep.2004.06.031. PMID 15507374.
- ↑ Rollinger, JM; Mocka, P; Zidorn, C; Ellmerer, EP; Langer, T; Stuppner, H (2005). "Application of the in combo screening approach for the discovery of non-alkaloid acetylcholinesterase inhibitors from Cichorium intybus". Current Drug Discovery Technologies 2 (3): 185–93. doi:10.2174/1570163054866855. PMID 16472227.
 |
