Chemistry:Sodium maleonitriledithiolate
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Disodium (Z)-1,2-dicyanoethene-1,2-bis(thiolate) | |
| Other names
Sodium mnt sodium maleonitriledithiolate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4N2Na2S2 | |
| Molar mass | 186.16 g·mol−1 |
| Appearance | yellow solid |
| Solubility in ethanol, DMF | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
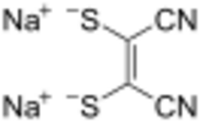
Sodium maleonitriledithiolate is the chemical compound described by the formula Na
2S
2C
2(CN)
2. The name refers to the cis compound, structurally related to maleonitrile ((CH(CN))
2). Maleonitriledithiolate is often abbreviated mnt. It is a "dithiolene", i.e. a chelating alkene-1,2-dithiolate. It is a prototypical non-innocent ligand in coordination chemistry. Several complexes are known, such as Ni(mnt)
2]2−.[2]:143–146
The salt is synthesized by treating carbon disulfide with sodium cyanide to give the cyanodithioformate salt, which eliminates elemental sulfur in aqueous solution:[3]
- 8 NaCN + 8 CS
2 → 4 Na
2S
2C
2(CN)
2 + S
8
The compound was first described by Bähr and Schleitzer 1958.[4]
References
- ↑ (in en) Chem Sources U.S.A.. Directories Publishing Company, Incorporated. 2001. p. 535. ISBN 978-0-937020-34-0. https://books.google.com/books?id=OxAaAQAAMAAJ.
- ↑ Day, Peter; Coronado, Eugenio (2004-12-14). Miller, Joel S.. ed (in en). Molecular Materials Combining Magnetic and Conducting Properties (1 ed.). Wiley. pp. 105–159. doi:10.1002/3527604383.ch4. ISBN 978-3-527-30665-7. https://onlinelibrary.wiley.com/doi/10.1002/3527604383.ch4.
- ↑ Davison, A.; Holm, R. H.; Benson, R. E.; Mahler, W. (January 1967). Muetterties, Earl L.. ed (in en). Metal Complexes Derived from cis -1,2-dicyano-1,2-ethylenedithiolate and Bis(Trifluoromethyl)-1,2-dithiete. 10 (1 ed.). Wiley. pp. 8–26. doi:10.1002/9780470132418.ch3. ISBN 978-0-470-13169-5. https://onlinelibrary.wiley.com/doi/10.1002/9780470132418.ch3.
- ↑ G. Bähr and G. Schleitzer (1957). "Beiträge zur Chemie des Schwefelkohlenstoffs und Selenkohlenstoffs, II. Die Kondensierende Spontan-Entschwefelung von Salzen und Estern der Cyan-Dithioameisensäure. Freie Cyan-Dithioameisensäure". Chemische Berichte 90 (3): 438–443. doi:10.1002/cber.19570900322.
 |


