Chemistry:Sodium metasilicate
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Sodium metasilicate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| Abbreviations | E550 |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | Sodium+metasilicate |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 1759 3253 |
| |
| |
| Properties | |
| Na2SiO3 | |
| Molar mass | 122.062 g·mol−1 |
| Appearance | White crystals |
| Density | 2.61 g/cm3 |
| Melting point | 1,088 °C (1,990 °F; 1,361 K) |
| 22.2 g/100 ml (25 °C) 160.6 g/100 ml (80 °C) | |
| Solubility | insoluble in alcohol |
Refractive index (nD)
|
1.52 |
| Thermochemistry | |
Heat capacity (C)
|
111.8 J/(K·mol) |
Std molar
entropy (S |
113.71 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−1561.43 kJ/mol |
Gibbs free energy (ΔfG˚)
|
−1427 kJ/mol |
| Hazards | |
| Safety data sheet | Avantor Performance Materials |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H314, H315, H319, H335 | |
| P260, P261, P264, P270, P271, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1153 (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
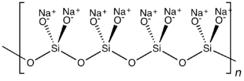
Sodium metasilicate is the chemical substance with formula Na2SiO3, which is the main component of commercial sodium silicate solutions. It is an ionic compound consisting of sodium cations Na+ and the polymeric metasilicate anions [–SiO2−3–]n. It is a colorless crystalline hygroscopic and deliquescent solid, soluble in water (giving an alkaline solution) but not in alcohols.[1]
Preparation and properties
The anhydrous compound can be prepared by fusing silicon dioxide SiO2 (silica, quartz) with sodium oxide Na2O in 1:1 molar ratio.[2]
The compound crystallizes from solution as various hydrates, such as
- pentahydrate Na2SiO3·5H2O (CAS 10213-79-3, EC 229-912-9, PubChem 57652358)
- nonahydrate Na2SiO3·9H2O (CAS 13517-24-3, EC 229-912-9, PubChem 57654617)[3]
Structure
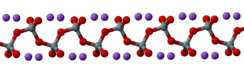
In the anhydrous solid, the metasilicate anion is actually polymeric, consisting of corner-shared {SiO4} tetrahedra, and not a discrete SiO32− ion.[4]
In addition to the anhydrous form, there are hydrates with the formula Na2SiO3·nH2O (where n = 5, 6, 8, 9), which contain the discrete, approximately tetrahedral anion SiO2(OH)22− with water of hydration. For example, the commercially available sodium silicate pentahydrate Na2SiO3·5H2O is formulated as Na2SiO2(OH)2·4H2O, and the nonahydrate Na2SiO3·9H2O is formulated as Na2SiO2(OH)2·8H2O.[5] The pentahydrate and nonahydrate forms have their own CAS Numbers, 10213-79-3 and 13517-24-3 respectively.
Uses
Sodium Metasilicate reacts with acids to produce silica gel.[6]
- Cements and Binders - dehydrated sodium metasilicate forms cement or binding agent.
- Pulp and Par - sizing agent and buffer/stabilizing agent when mixed with hydrogen peroxide.
- Soaps and Detergents - as an emulsifying and suspension agent.
- Automotive applications - decommissioning of old engines (CARS program), cooling system sealant, exhaust repair.
- Egg Preservative - seals eggs increasing shelf life.
- Crafts - forms "stalagmites" by reacting with and precipitating metal ions. Also used as a glue called "soluble glass".
- Hair coloring kits
See also
- Potassium metasilicate
- Sodium orthosilicate
- Sodium pyrosilicate
References
- ↑ Chemical Book: "Sodium metasilicate". Accessed on 2018-05-13.
- ↑ J. F. Schairer and N. L. Bowen (1956): "The system Na2O—Al2O3—SiO2". American Journal of Science, volume 254, issue 3, pages 129-195 doi:10.2475/ajs.254.3.129
- ↑ M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange" Journal of Physical Chemistry, volume 59, issue 6, pages 532–541. doi:10.1021/j150528a013
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN:0-19-855370-6
- ↑ "Uses of Sodium Metasilicate". https://sciencing.com/uses-sodium-metasilicate-5447484.html.
 |


