Chemistry:Solvothermal synthesis
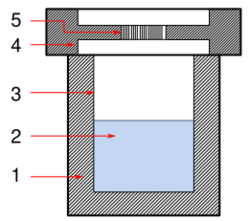
Solvothermal synthesis is a method of producing chemical compounds, in which a solvent containing reagents is put under high pressure and temperature in an autoclave. Many substances dissolve better in the same solvent in such conditions than at standard conditions, enabling reactions that would not otherwise occur and leading to new compounds or polymorphs. Solvothermal synthesis is very similar to the hydrothermal route; both are typically conducted in a stainless steel autoclave. The only difference being that the precursor solution is usually non-aqueous.[1]
Solvothermal synthesis has been used prepare MOFs,[2][3] titanium dioxide,[4] and graphene,[5] carbon spheres,[6] chalcogenides[7] and other materials.
Solvents
Besides water (hydrothermal synthesis), solvothermal syntheses make use of a large range of solvents, including ammonia, carbon dioxide, dimethylformamide, and various alcohols such as methanol, or glycols such as hexane-1,6-diol.[1][8][9]
Formic acid as reaction medium
Formic acid decomposes at high temperatures to carbon dioxide and hydrogen or carbon monoxide and water. This property allows formic acid to be used as a reducing and carbon dioxide-rich reaction medium in which it is possible to form various oxides and carbonates.[8]
Ammonia as reaction medium
The critical temperature and pressure of ammonia are 132.2 °C and 111 bar. In these conditions, it is possible to obtain a range of amides, imides, and nitrides. Although its dielectric constant is lower than that of water, ammonia behaves as a polar solvent especially at high pressures.[8]
References
- ↑ 1.0 1.1 Demazeau, G. (2008). "Solvothermal reactions: an original route for the synthesis of novel materials". J. Mater. Sci. 43 (7): 2104–2114. doi:10.1007/s10853-007-2024-9. Bibcode: 2008JMatS..43.2104D.
- ↑ Yaghi, O. M.; Kalmutzki, M. J.; Diercks, C. S. (2019). Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks. Weinheim: Wiley-VCH. ISBN 978-3-527-82110-5.
- ↑ Farha, Omar K.; Hupp, Joseph T. (2010). "Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials". Accounts of Chemical Research 43 (8): 1166–1175. doi:10.1021/ar1000617. PMID 20608672.
- ↑ Ong, Wee-Jun; Tan, Lling-Lling; Chai, Siang-Piao; Yong, Siek-Ting; Mohamed, Abdul Rahman (2014). "Highly reactive {001} facets of TiO2-based composites: Synthesis, formation mechanism and characterization". Nanoscale 6 (4): 1946–2008. doi:10.1039/c3nr04655a. PMID 24384624. Bibcode: 2014Nanos...6.1946O.
- ↑ Xiang, Quanjun; Yu, Jiaguo; Jaroniec, Mietek (2012). "Graphene-based semiconductor photocatalysts". Chem. Soc. Rev. 41 (2): 782–796. doi:10.1039/C1CS15172J. PMID 21853184.
- ↑ Hu, Gang; Ma, Ding; Cheng, Mojie; Liu, Lin; Bao, Xinhe (2002). "Direct synthesis of uniform hollow carbon spheres by a self-assembly template approach". Chemical Communications (17): 1948–1949. doi:10.1039/B205723A. PMID 12271688.
- ↑ Li, J.; Chen, Z.; Wang, R. J.; Proserpio, D. M. (1999). "Low temperature route towards new materials: Solvothermal synthesis of metal chalcogenides in ethylenediamine". Coordination Chemistry Reviews 190-192: 707–735. doi:10.1016/S0010-8545(99)00107-1.
- ↑ 8.0 8.1 8.2 Rabenau, Albrecht (1985). "The Role of Hydrothermal Synthesis in Preparative Chemistry" (in en). Angewandte Chemie International Edition in English 24 (12): 1026–1040. doi:10.1002/anie.198510261. ISSN 0570-0833. https://onlinelibrary.wiley.com/doi/10.1002/anie.198510261.
- ↑ Springer handbook of crystal growth. Govindhan Dhanaraj. Heidelberg: Springer. 2010. ISBN 978-3-540-74761-1. OCLC 671821738. https://www.worldcat.org/oclc/671821738.
 |
