Chemistry:Divergent synthesis
In chemistry a divergent synthesis is a strategy with the aim to improve the efficiency of chemical synthesis. It is often an alternative to convergent synthesis or linear synthesis.
In one strategy divergent synthesis aims to generate a library of chemical compounds by first reacting a molecule with a set of reactants. The next generation of compounds is generated by further reactions with each compound in generation 1. This methodology quickly diverges to large numbers of new compounds
- A generates A1, A2, A3, A4, A5 in generation 1
- A1 generates A11, A12, A13 in generation 2 and so on.
An entire library of new chemical compounds, for instance saccharides, can be screened for desirable properties. In another strategy divergent synthesis starts from a molecule as a central core from which successive generations of building blocks are added. A good example is the divergent synthesis of dendrimers, for example, where in each generation a new monomer reacts to the growing surface of the sphere.
Diversity oriented synthesis
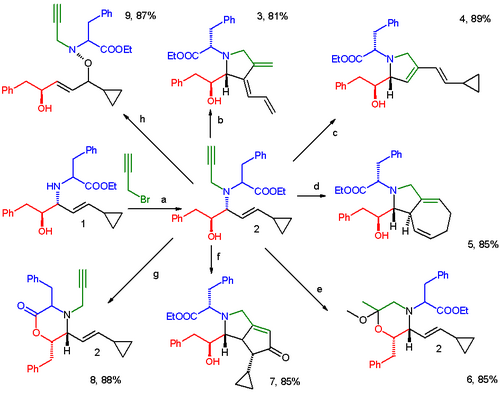
Diversity oriented synthesis or DOS is a strategy for quick access to molecule libraries with an emphasis on skeletal diversity.[1] In one such application a Petasis reaction product (1) is functionalized with propargyl bromide leading to a starting compound (2) having 5 functional groups.[2] This molecule can be subjected to a range of reagents yielding unique molecular skeletons in one generation.[3]
DOS Drugs
- Dosabulin
- Gemmacin B
- ML238
- Robotnikinin
References
- ↑ Burke, Martin; Schreiber, Stuart (2004). "A Planning Strategy for Diversity-Oriented Synthesis". Angewandte Chemie International Edition 43 (1): 46–58. doi:10.1002/anie.200300626. PMID 14694470.
- ↑ Short Synthesis of Skeletally and Stereochemically Diverse Small Molecules by Coupling Petasis Condensation Reactions to Cyclization Reactions Naoya Kumagai, Giovanni Muncipinto, Stuart L. Schreiber Angewandte Chemie International Edition Volume 45, Issue 22 , Pages 3635 - 3638 2006 Abstract
- ↑ Path b to 3: cycloisomerization with Pd(PPh3)2(OAc)2. Path c to 4: enyne metathesis with Hoveyda–Grubbs catalyst. Path d to 5: CpRu(CH3CN)3PF6 initiated [5+2]cycloaddition. Path e to 6: Alkyne hydrolysis with NaAuCl4 in MeOH. Path f to 7: Pauson–Khand reaction with Co2(CO)8. Path g to 8: Esterification with sodium hydride. Path h to 9: Oxidation with mCPBA
 |