Chemistry:Speciociliatine
From HandWiki
Short description: Chemical compound
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
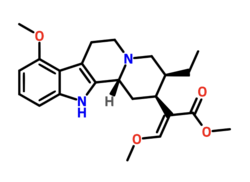
| Formula | C23H30N2O4 |
| Molar mass | 398.503 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 104 °C[1] |
| |
| |
Speciociliatine is a major alkaloid of the plant Mitragyna speciosa, commonly known as kratom. It is a stereoisomer of Mitragynine and constitutes 0.00156 - 2.9% of the dried leaf material.[2][3]
Pharmacology
Pharmacodynamics
Speciociliatine has found to be a ligand of the mu and kappa opioid receptors, however findings are varied as to whether it functions as an agonist or a competitive antagonist at those sites.[4][5]
Pharmacokinetics
A preliminary pharmacokinetic analysis in male Sprague Dawley rats determined the elimination half-life of Speciociliatine to be 2.6 - 5 hours and the absolute bioavailability to be 20.7% (at an oral dose of 20 mg/kg).[6]
References
- ↑ "National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 15560576, Speciociliatine.". https://pubchem.ncbi.nlm.nih.gov/compound/Speciociliatine.
- ↑ "Kratom (Mitragyna speciosa) Validation: Quantitative Analysis of Indole and Oxindole Alkaloids Reveals Chemotypes of Plants and Products", Planta Medica (Georg Thieme Verlag KG) 88 (9/10): 838–857, 2022, doi:10.1055/a-1795-5876, PMID 35468648, PMC 9343938, http://dx.doi.org/10.1055/a-1795-5876
- ↑ "Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra-performance liquid chromatography−tandem mass spectrometry", Drug Testing and Analysis (Wiley) 11 (8): 1162–1171, 2019, doi:10.1002/dta.2604, PMID 30997725, PMC 7927418, http://dx.doi.org/10.1002/dta.2604
- ↑ "Investigation of the Adrenergic and Opioid Binding Affinities, Metabolic Stability, Plasma Protein Binding Properties, and Functional Effects of Selected Indole-Based Kratom Alkaloids", Journal of Medicinal Chemistry (American Chemical Society (ACS)) 63 (1): 433–439, 2019, doi:10.1021/acs.jmedchem.9b01465, PMID 31834797, PMC 7676998, http://dx.doi.org/10.1021/acs.jmedchem.9b01465
- ↑ "Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators", Journal of the American Chemical Society (American Chemical Society (ACS)) 138 (21): 6754–6764, 2016, doi:10.1021/jacs.6b00360, PMID 27192616, PMC 5189718, http://dx.doi.org/10.1021/jacs.6b00360
- ↑ "Preclinical pharmacokinetic study of speciociliatine, a kratom alkaloid, in rats using an UPLC-MS/MS method", Journal of Pharmaceutical and Biomedical Analysis (Elsevier BV) 194: 113778, 2021, doi:10.1016/j.jpba.2020.113778, PMID 33277117, http://dx.doi.org/10.1016/j.jpba.2020.113778
 |

