Chemistry:Sulfamide
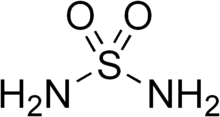
| |
| |
| Names | |
|---|---|
| IUPAC name
Sulfuric diamide
| |
| Preferred IUPAC name
Sulfamide | |
| Other names
Sulphamide
Sulfuryl amide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| H 4N 2O 2S | |
| Molar mass | 96.11 g/mol |
| Appearance | White orthorhombic plates |
| Melting point | 93 °C (199 °F; 366 K) |
| Boiling point | 250 °C (482 °F; 523 K) (decomposes) |
| Freely soluble | |
| -44.4×10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
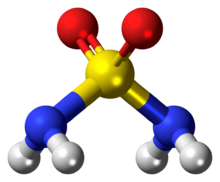
Sulfamide (IUPAC name: sulfuric diamide) is a compound with the chemical formula SO
2(NH
2)
2 and structure H
2N–S(=O)
2–NH
2. Sulfamide is produced by the reaction of sulfuryl chloride with ammonia. Sulfamide was first prepared in 1838 by the French chemist Henri Victor Regnault.[2]
Sulfamide functional group
In organic chemistry, the term sulfamide may also refer to the functional group which consists of at least one organic group attached to a nitrogen atom of sulfamide.
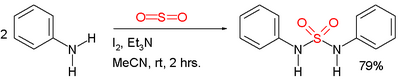
Symmetric sulfamides can be prepared directly from amines, sulfur dioxide gas and an oxidant:[3]
In this example, the reactants are aniline, triethylamine (Et
3N, Et = ethyl group), and iodine. Sulfur dioxide is believed to be activated through a series of intermediates: Et
3N–+
–I−
, Et
3N–I+
–I−
3 and Et
3N+
–SO−
2.
The sulfamide functional group is an increasingly common structural feature used in medicinal chemistry.[4]
See also
References
- ↑ Merck Index, 11th Edition, 8894.
- ↑ Regnault, Victor (1838) "Sur l'acide chlorosulfurique et la sulfamide" (On sulfuryl chloride and sulfamide), Annales de chimie et de physique, series 2, 69 : 170-184; see especially "Action de gaz ammoniac sec sur la liqueur chlorosulfurique" (Action of dry ammonia gas on liquid sulfuryl chloride), pages 176-180.
- ↑ Leontiev, A. V.; Dias, H. V. R.; Rudkevich, D. M. (2006). "Sulfamides and sulfamide polymers directly from sulfur dioxide". Chemical Communications 2006 (27): 2887–2889. doi:10.1039/b605063h. PMID 17007406.
- ↑ Reitz, A. B.; Smith, G. R.; Parker, M. H. (2009). "The Role of Sulfamide Derivatives in Medicinal Chemistry: A Patent Review (2006 – 2008)". Expert Opinion on Therapeutic Patents 19 (10): 1449–1453. doi:10.1517/13543770903185920. PMID 19650745.
 |