Chemistry:Sulfluramid
From HandWiki
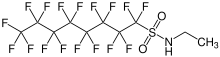
| |
| Names | |
|---|---|
| IUPAC name
N-ethyl-1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctane-1-sulfonamide
| |
| Other names
N-Ethylperfluorooctylsulfonamide
N-Ethylheptadecafluorooctanesulfonamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H6F17NO2S | |
| Molar mass | 527.20 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfluramid (N-EtFOSA) is a chemical compound from the group of sulfonic acid amides and per- and polyfluoroalkyl substances (PFASs) that is effective as an insecticide.
Annual production increased from about 30 tons in 2003 to 60 tons in 2013.[1]
Environmental issues
It is predominantly biotransformed to perfluorooctanesulfonic acid (PFOS),[2] but partly also to perfluorooctanoic acid (PFOA).[3][4] Sulfluramid benefits from an acceptable purpose in the listing of PFOS to annex B of the Stockholm Convention on Persistent Organic Pollutants.

Metabolic pathways for the biotransformation of sulfluramid to PFOS[5]
References
- ↑ Löfstedt Gilljam, John; Leonel, Juliana; Cousins, Ian T.; Benskin, Jonathan P. (2016-01-19). "Is Ongoing Sulfluramid Use in South America a Significant Source of Perfluorooctanesulfonate (PFOS)? Production Inventories, Environmental Fate, and Local Occurrence" (in en). Environmental Science & Technology 50 (2): 653–659. doi:10.1021/acs.est.5b04544. PMID 26653085.
- ↑ "Sulfluramid Registration Review Final Decision; Notice of Availability". 2008. https://www.federalregister.gov/documents/2008/10/29/E8-25667/sulfluramid-registration-review-final-decision-notice-of-availability.
- ↑ Plumlee, Megan H.; McNeill, Kristopher; Reinhard, Martin (2009-05-15). "Indirect Photolysis of Perfluorochemicals: Hydroxyl Radical-Initiated Oxidation of N-Ethyl Perfluorooctane Sulfonamido Acetate (N-EtFOSAA) and Other Perfluoroalkanesulfonamides" (in en). Environmental Science & Technology 43 (10): 3662–3668. doi:10.1021/es803411w. PMID 19544870. Bibcode: 2009EnST...43.3662P.
- ↑ Liu, Zhaoyang; Lu, Yonglong; Wang, Pei; Wang, Tieyu; Liu, Shijie; Johnson, Andrew C.; Sweetman, Andrew J.; Baninla, Yvette (February 2017). "Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China" (in en). Science of the Total Environment 580: 1247–1256. doi:10.1016/j.scitotenv.2016.12.085. PMID 28040212. Bibcode: 2017ScTEn.580.1247L. http://ir.rcees.ac.cn/handle/311016/39071.
- ↑ Zhang, Wenping; Pang, Shimei; Lin, Ziqiu; Mishra, Sandhya; Bhatt, Pankaj; Chen, Shaohua (March 2021). "Biotransformation of perfluoroalkyl acid precursors from various environmental systems: advances and perspectives" (in en). Environmental Pollution 272: 115908. doi:10.1016/j.envpol.2020.115908. PMID 33190976.
 |

