Chemistry:Tellimagrandin I
| |
| Names | |
|---|---|
| Systematic IUPAC name
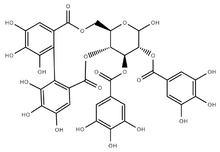
(11aR,13Ξ,14R,15S,15aR)-2,3,4,5,6,7,13-Heptahydroxy-9,17-dioxo-9,11,11a,13,14,15,15a,17-octahydrodibenzo[g,i]pyrano[3,2-b][1,5]dioxacycloundecine-14,15-diyl bis(3,4,5-trihydroxybenzoate) | |
| Other names
1-desgalloyleugeniin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C34H26O22 | |
| Molar mass | 786.56 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tellimagrandin I is an ellagitannin found in plants, such as Cornus canadensis, Eucalyptus globulus, Melaleuca styphelioides, Rosa rugosa, and walnut. It is composed of two galloyl and one hexahydroxydiphenyl groups bound to a glucose residue. It differs from Tellimagrandin II only by a hydroxyl group instead of a third galloyl group. It is also structurally similar to punigluconin and pedunculagin, two more ellagitannin monomers.
Tellimagrandin I has been shown to restore antioxidant enzyme activity in glucose- and oxalate-challenged rat cells[1] and affects Cu(II)- and Fe(II)-dependent DNA strand breaks.[2][3] It has hepatoprotective effects on carbon tetrachloride- and d-galactosamine-stressed HepG2 cells[4][5] and enhances peroxisomal fatty acid beta-oxidation in liver, increasing mRNA expression of PPAR alpha, ACOX1, and CPT1A.[6] It enhances gap junction communication and reduces tumor phenotype in HeLa cells[7] and inhibits invasion of HSV-1[8] and HCV similar to eugeniin and casuarictin.[9]
See also
- Ellagitannin
- Pedunculagin
- Punigluconin
- Tellimagrandin II
References
- ↑ "Antioxidant activity of phenolic compounds from extracts of Eucalyptus globulus and Melaleuca styphelioides and their protective role on D-glucose-induced hyperglycemic stress and oxalate stress in NRK-49Fcells". Nat Prod Res 32 (11): 1274–1280. 21 Jun 2017. doi:10.1080/14786419.2017.1343324. PMID 28637361. https://figshare.com/articles/journal_contribution/5135008.
- ↑ "Chebulinic acid and tellimagrandin I inhibit DNA strand breaks by hydroquinone/Cu(II) and H(2)O(2)/Cu(II), but potentiate DNA strand breaks by H(2)O(2)/Fe(II)". Toxicol in Vitro 23 (4): 667–673. Jun 2009. doi:10.1016/j.tiv.2009.03.009. PMID 19328845.
- ↑ "Prooxidant action of chebulinic acid and tellimagrandin I: causing copper-dependent DNA strand breaks". Toxicology in Vitro 23 (3): 425–431. Apr 2009. doi:10.1016/j.tiv.2009.01.012. PMID 19344683.
- ↑ "Hepatoprotective and antioxidant effect of ellagitannins and galloyl esters isolated from Melaleuca styphelioides on carbon tetrachloride-induced hepatotoxicity in HepG2 cells". Pharm Biol 54 (9): 1727–1735. Sep 2016. doi:10.3109/13880209.2015.1125933. PMID 26731241.
- ↑ "Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine: hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut". J Agric Food Chem 56 (12): 4444–4449. 25 Jun 2008. doi:10.1021/jf8002174. PMID 18494495.
- ↑ "Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver". J Agric Food Chem 57 (5): 1786–1792. 11 Mar 2009. doi:10.1021/jf803441c. PMID 19256553.
- ↑ "Tellimagrandin I enhances gap junctional communication and attenuates the tumor phenotype of human cervical carcinoma HeLa cells in vitro". Cancer Lett 242 (1): 77–87. 8 Oct 2006. doi:10.1016/j.canlet.2005.10.044. PMID 16338066.
- ↑ "Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis". BMC Complement Altern Med 17 (1): 123. 22 Feb 2017. doi:10.1186/s12906-017-1618-2. PMID 28228101.
- ↑ "Tellimagrandin I, HCV invasion inhibitor from Rosae Rugosae Flos". Bioorg Med Chem Lett 20 (5): 1598–1600. 2010. doi:10.1016/j.bmcl.2010.01.084. PMID 20144544.
 |

