Chemistry:Temperature-responsive polymer
Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature.[1] The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive (for short, thermosensitive) materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.
Research mainly focuses on polymers that show thermoresponsivity in aqueous solution. Promising areas of application are tissue engineering,[2] liquid chromatography,[3][4] drug delivery[2][5] and bioseparation.[6] Only a few commercial applications exist, for example, cell culture plates coated with an LCST-polymer.
History
The theory of thermoresponsive polymer (similarly, microgels) begins in the 1940s with work from Flory and Huggins who both independently produced similar theoretical expectations for polymer in solution with varying temperature.
The effects of external stimuli on particular polymers were investigated in the 1960s by Heskins and Guillet.[7] They established 32°C as the lower critical solution temperature (LCST) for poly(N-isopropylacrylamide).
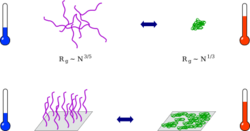
Coil-globule transition
Thermoresponsive polymer chains in solution adopt an expanded coil conformation. At the phase separation temperature they collapse to form compact globuli. This process can be observed directly by methods of static and dynamic light scattering.[8][9] The drop in viscosity can be indirectly observed. When mechanisms which reduce surface tension are absent, the globules aggregate, subsequently causing turbidity and the formation of visible particles.
Phase diagrams of thermoresponsive polymers
The phase separation temperature (and hence, the cloud point) is dependent on polymer concentration. Therefore, temperature-composition diagrams are used to display thermoresponsive behavior over a wide range of concentrations.[10] Phases separate into a polymer-poor and a polymer-rich phase. In strictly binary mixtures the composition of the coexisting phases can be determined by drawing tie-lines. However, since polymers display a molar mass distribution this straightforward approach may be insufficient.
During the process of phase separation the polymer-rich phase can vitrify before equilibrium is reached. This depends on the glass transition temperature for each individual composition. It is convenient to add the glass transition curve to the phase diagram, although it is no real equilibrium. The intersection of the glass transition curve with the cloud point curve is called Berghmans point.[11] In the case of UCST polymers, above the Berghmans point the phases separate into two liquid phases, below this point into a liquid polymer-poor phase and a vitrified polymer-rich phase. For LCST polymers the inverse behavior is observed.
Thermodynamics
Polymers dissolve in a solvent when the Gibbs energy of the system decreases, i.e., the change of Gibbs energy (ΔG) is negative. From the known Legendre transformation of the Gibbs–Helmholtz equation it follows that ΔG is determined by the enthalpy of mixing (ΔH) and entropy of mixing (ΔS).
Without interactions between the compounds there would be no enthalpy of mixing and the entropy of mixing would be ideal. The ideal entropy of mixing of multiple pure compounds is always positive (the term -T∙ΔS is negative) and ΔG would be negative for all compositions, causing complete miscibility. Therefore, the fact that miscibility gaps are observed can only be explained by interaction. In the case of polymer solutions, polymer-polymer, solvent-solvent and polymer-solvent interactions have to be taken into account. A model for the phenomenological description of polymer phase diagrams was developed by Flory and Huggins (see Flory–Huggins solution theory). The resulting equation for the change of Gibbs energy consists of a term for the entropy of mixing for polymers and an interaction parameter that describes the sum of all interactions.[11]File:Temperature dependence of interaction parameter x.tif
where
- R = universal gas constant
- m = number of occupied lattice sites per molecule (for polymer solutions m1 is approximately equal to the degree of polymerization and m2=1)
- φ = volume fraction of the polymer and the solvent, respectively
- χ = interaction parameter
A consequence of the Flory-Huggins theory is, for instance, that the UCST (if it exists) increases and shifts into the solvent-rich region when the molar mass of the polymer increases. Whether a polymer shows LCST and/or UCST behavior can be derived from the temperature-dependence of the interaction parameter (see figure). The interaction parameter not only comprises enthalpic contributions but also the non-ideal entropy of mixing, which again consists of many individual contributions (e.g., the strong hydrophobic effect in aqueous solutions). For these reasons, classical Flory-Huggins theory cannot provide much insight into the molecular origin of miscibility gaps.
Applications
Bioseparation
Thermoresponsive polymers can be functionalized with moieties that bind to specific biomolecules. The polymer-biomolecule conjugate can be precipitated from solution by a small change of temperature.[6][12] Isolation may be achieved by filtration or centrifugation.
Thermoresponsive surfaces
Tissue engineering
For some polymers it was demonstrated that thermoresponsive behavior can be transferred to surfaces. The surface is either coated with a polymer film or the polymer chains are bound covalently to the surface. This provides a way to control the wetting properties of a surface by small temperature changes. The described behavior can be exploited in tissue engineering since the adhesion of cells is strongly dependent on the hydrophilicity/hydrophobicity.[2][13] This way, it is possible to detach cells from a cell culture dish by only small changes in temperature, without the need to additionally use enzymes (see figure). Respective commercial products are already available.
Chromatography
Thermoresponsive polymers can be used as the stationary phase in liquid chromatography.[3] Here, the polarity of the stationary phase can be varied by temperature changes, altering the power of separation without changing the column or solvent composition. Thermally related benefits of gas chromatography can now be applied to classes of compounds that are restricted to liquid chromatography due to their thermolability. In place of solvent gradient elution, thermoresponsive polymers allow the use of temperature gradients under purely aqueous isocratic conditions.[14] The versatility of the system is controlled not only by changing temperature, but also by adding modifying moieties that allow for a choice of enhanced hydrophobic interaction, or by introducing the prospect of electrostatic interaction.[15] These developments have already brought major improvements to the fields of hydrophobic interaction chromatography, size exclusion chromatography, ion exchange chromatography, and affinity chromatography separations, as well as pseudo-solid phase extractions ("pseudo" because of phase transitions).
Thermoresponsive gels
Covalently linked gels
Three-dimensional covalently linked polymer networks are insoluble in all solvents, they merely swell in good solvents.[16][17] Thermoresponsive polymer gels show a discontinuous change of the degree of swelling with temperature. At the volume phase transition temperature (VPTT) the degree of swelling changes drastically. Researchers try to exploit this behavior for temperature-induced drug delivery. In the swollen state, previously incorporated drugs are released easily by diffusion.[18] More sophisticated "catch and release" techniques have been elaborated in combination with lithography[19] and molecular imprinting.[20]
Physical gels
In physical gels unlike covalently linked gels the polymers chains are not covalently linked together. That means that the gel could re-dissolve in a good solvent under some conditions. Thermoresponsive physical gels, also sometimes called thermoresponsive injectable gels have been used in Tissue Engineering.[21][22][23][2][24] This involves mixing at room temperature the thermoresponsive polymer in solution with the cells and then inject the solution to the body. Due to the temperature increase (to body temperature) the polymer creates a physical gel. Within this physical gel the cells are encapsulated. Tailoring the temperature that the polymer solution gels can be challenging because this depend by many factors like the polymer composition,[25][26][27][28] architecture[25][26] as well as the molar mass.[27]
Thermoreversible materials
Some thermoreversible gels are used in biomedicine. For instance, hydrogels made of proteins are used as scaffolds in knee replacement.[29] In baking, thermoreversible glazes such as pectin are prized for their ability to set and then reset after melting,[30] and are used in nappage and other processes to ensure a smooth final surface for a presented dish.[31][32] In manufacturing, thermoplastic elastomers can be set into a shape and then reset to their original shape through thermal reversibility, unlike one-way thermoset elastomers.[33]
Characterization of thermoresponsive polymer solutions
Cloud point
Experimentally, the phase separation can be followed by turbidimetry. There is no universal approach for determining the cloud point suitable for all systems. It is often defined as the temperature at the onset of cloudiness, the temperature at the inflection point of the transmittance curve, or the temperature at a defined transmittance (e.g., 50%).[11] The cloud point can be affected by many structural parameters of the polymer like the hydrophobic content,[25][26][27][28][34] architecture[25][26] and even the molar mass.[27][35]
Hysteresis
The cloud points upon cooling and heating of a thermoresponsive polymer solution do not coincide because the process of equilibration takes time. The temperature interval between the cloud points upon cooling and heating is called hysteresis. The cloud points are dependent on the cooling and heating rates, and hysteresis decreases with lower rates. There are indications that hysteresis is influenced by the temperature, viscosity, glass transition temperature and the ability to form additional intra- and inter-molecular hydrogen bonds in the phase separated state.[36]
Other properties
Another important property for potential applications is the extent of phase separation, represented by the difference in polymer content in the two phases after phase separation. For most applications, phase separation in pure polymer and pure solvent would be desirable although it is practically impossible. The extent of phase separation in a given temperature interval depends on the particular polymer-solvent phase diagram.
Example: From the phase diagram of polystyrene (molar mass 43,600 g/mol) in the solvent cyclohexane it follows that at a total polymer concentration of 10%, cooling from 25 to 20 °C causes phase separation into a polymer-poor phase with 1% polymer and a polymer-rich phase with 30% polymer content.[37]
Also desirable for many applications is a sharp phase transition, which is reflected by a sudden drop in transmittance. The sharpness of the phase transition is related to the extent of phase separation but additionally relies on whether all present polymer chains exhibit the same cloud point. This depends on the polymer endgroups, dispersity, or—in the case of copolymers—varying copolymer compositions.[36] As a result of phase separation, thermoresponsive polymer systems can form well-defined self-assembled nanostructures with a number of different practical application such as in drug and gene delivery, tissue engineering, etc. In order to establish the required properties for applications, a rigorous characterization of the phase separation phenomenon can be carried out by different spectroscopic and calorimetric methods, including nuclear magnetic resonance (NMR) , dynamic light scattering (DLS), small-angle X-ray scattering (SAXS), infrared spectroscopy (IR), Raman spectroscopy, and Differential scanning calorimetry (DSC).[38]
Examples of thermoresponsive polymers
Thermoresponsivity in organic solvents
Due to the low entropy of mixing, miscibility gaps are often observed for polymer solutions.[11] Many polymers are known that show UCST or LCST behavior in organic solvents.[39] Examples for organic polymer solutions with UCST are polystyrene in cyclohexane,[37][40] polyethylene in diphenylether[41][42] or polymethylmethacrylate in acetonitrile.[43] An LCST is observed for, e.g., polypropylene in n-hexane,[44] polystyrene in butylacetate[45] or polymethylmethacrylate in 2-propanone.[46]
Thermoresponsivity in water
Polymer solutions that show thermoresponsivity in water are especially important since water as a solvent is cheap, safe and biologically relevant. Current research efforts focus on water-based applications like drug delivery systems, tissue engineering, bioseparation (see the section Applications). Numerous polymers with LCST in water are known.[11] The most studied polymer is poly(N-isopropylacrylamide).[47][48][49] Further examples are poly[2-(dimethylamino)ethyl methacrylate] (pDMAEMA)[25][26][27][28][35] hydroxypropylcellulose,[50] ,[51] poly-2-isopropyl-2-oxazoline[52] and polyvinyl methyl ether.[53]
Some industrially relevant polymers show LCST as well as UCST behavior whereas the UCST is found outside the 0-to-100 °C region and can only be observed under extreme experimental conditions.[36] Examples are polyethylene oxide,[54][55][56] polyvinylmethylether[57] and polyhydroxyethylmethacrylate.[58] There are also polymers that exhibit UCST behavior between 0 and 100 °C. However, there are large differences concerning the ionic strength at which UCST behavior is detected. Some zwitterionic polymers show UCST behavior in pure water and also in salt-containing water or even at higher salt concentration.[59][60][61][62] By contrast, polyacrylic acid displays UCST behavior solely at high ionic strength.[63] Examples for polymer that show UCST behavior in pure water as well as under physiological conditions are poly(N-acryloylglycinamide),[64][65][66] ureido-functionalized polymers,[67] copolymers from N-vinylimidazole and 1-vinyl-2-(hydroxylmethyl)imidazole[68] or copolymers from acrylamide and acrylonitrile.[69] Polymers for which UCST relies on non-ionic interactions are very sensitive to ionic contamination. Small amounts of ionic groups may suppress phase separation in pure water.
The UCST is dependent on the molecular mass of the polymer. For the LCST this is not necessarily the case, as shown for poly(N-isopropylacrylamide).[70][71][72]
Schizophrenic behavior of UCST-LCST diblock copolymers
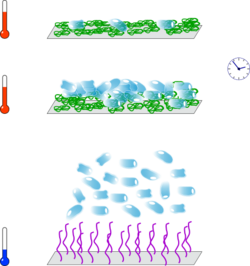
A more complex scenario can be found in the case of diblock copolymers that feature two orthogonally thermo-responsive blocks, i.e., an UCST and an LCST-type block. By applying a temperature stimulus, the individual polymer blocks show different phase transitions, e.g. by increasing the temperature, the UCST-type block features an insoluble-soluble transition, while the LCST-type block undergoes a soluble-insoluble transition.[73][74][75] The order of the individual phase transitions depends on the relative positions of the UCST and LCST. Thus, upon temperature change the roles of the soluble and insoluble polymer blocks are reversed and this structural inversion is typically called 'schizophrenic' in the literature.[76][77][78] Besides the fundamental interest in the mechanism of this behavior, such block copolymers have been proposed for application in smart emulsification, drug delivery, and rheology control.[79][80][81] Schizophrenic diblock copolymer have also been applied as thin films for potential use as sensors, smart coatings or nanoswitches, and soft robotics.[82][83][84][85][59]
References
- ↑ Hoffman, Allan S. (May 1995). ""Intelligent" Polymers in Medicine and Biotechnology". Artificial Organs 19 (5): 458–467. doi:10.1111/j.1525-1594.1995.tb02359.x. PMID 7625927. https://onlinelibrary.wiley.com/doi/10.1111/j.1525-1594.1995.tb02359.x.
- ↑ 2.0 2.1 2.2 2.3 Ward, Mark A.; Georgiou, Theoni K. (August 3, 2011). "Thermoresponsive Polymers for Biomedical Applications". Polymers 3 (3): 1215–1242. doi:10.3390/polym3031215. ISSN 2073-4360.
- ↑ 3.0 3.1 Tan, Irene; Roohi, Farnoosh; Titirici, Maria-Magdalena (October 19, 2011). "Thermoresponsive polymers in liquid chromatography". Analytical Methods 4 (1): 34–43. doi:10.1039/C1AY05356F. ISSN 1759-9679. https://pubs.rsc.org/en/content/articlelanding/2012/ay/c1ay05356f. Retrieved October 4, 2022.
- ↑ Maharjan, Pankaj; Woonton, Brad W.; Bennett, Louise E.; Smithers, Geoffrey W.; DeSilva, Kirthi; Hearn, Milton T. W. (2008-04-01). "Novel chromatographic separation — The potential of smart polymers". Innovative Food Science & Emerging Technologies. Food Innovation: Emerging Science, Technologies and Applications (FIESTA) Conference (New York: Elsevier Science) 9 (2): 232–242. doi:10.1016/j.ifset.2007.03.028. ISSN 1466-8564. https://www.sciencedirect.com/science/article/pii/S1466856407001233.
- ↑ Bajpai, A. K.; Shukla, Sandeep K.; Bhanu, Smitha; Kankane, Sanjana (November 2008). "Responsive polymers in controlled drug delivery". Progress in Polymer Science 33 (11): 1088–1118. doi:10.1016/j.progpolymsci.2008.07.005. ISSN 0079-6700. https://www.sciencedirect.com/science/article/pii/S0079670008000609. Retrieved 2022-10-04.
- ↑ 6.0 6.1 Galaev, Igor; Mattiasson, Bo (November 15, 2001). Smart Polymers for Bioseparation and Bioprocessing. Taylor & Francis. ISBN 978-1-4398-5816-5. OCLC 54395177. https://books.google.com/books?id=MKg9crX8eusC.
- ↑ Michael Heskins; James E. Guillet (1968). "Solution Properties of Poly(N-isopropylacrylamide)". J. Macromol. Sci. Chem. 2 (8): 1441–1455. doi:10.1080/10601326808051910.
- ↑ Wu, Chi; Wang, Xiaohui (1998-05-04). "Globule-to-Coil Transition of a Single Homopolymer Chain in Solution". Physical Review Letters 80 (18): 4092–4094. doi:10.1103/PhysRevLett.80.4092. Bibcode: 1998PhRvL..80.4092W. https://link.aps.org/doi/10.1103/PhysRevLett.80.4092.
- ↑ Vshivkov, Sergei A.; Safronov, Alexander P. (November 1997). "The conformational coil-globule transition of polystyrene in cyclohexane solution". Macromolecular Chemistry and Physics 198 (10): 3015–3023. doi:10.1002/macp.1997.021981003. https://onlinelibrary.wiley.com/doi/10.1002/macp.1997.021981003.
- ↑ Koningsveld, R. (2001). Polymer Phase Diagrams: A Textbook. W. H. Stockmayer, E. Nies. Oxford, UK: Oxford University Press. ISBN 978-0-19-855635-0. OCLC 45375807. https://books.google.com/books?id=agwpOfP2zI4C.
- ↑ 11.0 11.1 11.2 11.3 11.4 Aseyev, Vladimir; Tenhu, Heikki; Winnik, Françoise M.; Müller; Borisov, Oleg (2011-09-15). "Non-ionic Thermoresponsive Polymers in Water". Self Organized Nanostructures of Amphiphilic Block Copolymers II. Berlin, Heidelberg: Springer. pp. 29–89. doi:10.1007/12_2010_57. ISBN 978-3-642-22297-9. OCLC 756912488. https://www.chemie.uni-bayreuth.de/mcii/de/pub/23615/Vol_242_Sample_pages.pdf. Retrieved 2022-10-29. on Google Books. link to original publication.
- ↑ Chen, J. P.; Hoffman, A. S. (November 1990). "Polymer-protein conjugates. II. Affinity precipitation separation of human immunogammaglobulin by a poly(N-isopropylacrylamide)-protein A conjugate". Biomaterials 11 (9): 631–634. doi:10.1016/0142-9612(90)90020-q. ISSN 0142-9612. PMID 2090296.
- ↑ Lee, EL.; von Recum, HA (2010). "Cell culture platform with mechanical conditioning and nondamaging cellular detachment". J Biomed Mater Res A 93 (2): 411–8. doi:10.1002/jbm.a.32754. PMID 20358641.
- ↑ Hideko Kanazawa (2007). "Thermally responsive chromatographic materials using functional polymers". J. Sep. Sci. 30 (11): 1646–1656. doi:10.1002/jssc.200700093. PMID 17623446.
- ↑ Eri Ayano; Hideko Kanazawa (2006). "Aqueous chromatography system using temperature-responsive polymer-modified stationary phases". J. Sep. Sci. 29 (6): 738–749. doi:10.1002/jssc.200500485. PMID 16830486.
- ↑ Patrickios, Costas S.; Georgiou, Theoni K. (2003-03-01). "Covalent amphiphilic polymer networks". Current Opinion in Colloid & Interface Science 8 (1): 76–85. doi:10.1016/S1359-0294(03)00005-0.
- ↑ Rikkou-Kalourkoti, M.; Patrickios, C. S.; Georgiou, T. K. (2012-01-01). Möller, Krzysztof MatyjaszewskiMartin. ed. 6.08 - Model Networks and Functional Conetworks. Amsterdam: Elsevier. pp. 293–308. doi:10.1016/b978-0-444-53349-4.00166-7. ISBN 978-0-08-087862-1.
- ↑ Dinarvand, R.; D'Emanuele, A. (1995-10-01). "The use of thermoresponsive hydrogels for on-off release of molecules". Journal of Controlled Release 36 (3): 221–227. doi:10.1016/0168-3659(95)00035-7. ISSN 0168-3659.
- ↑ Alexandro Castellanos; Samuel J. DuPont; August J. Heim II; Garrett Matthews; Peter G. Stroot; Wilfrido Moreno; Ryan G. Toomey (2007). "Size-Exclusion "capture and release" separations using surface-patterned poly(N-isopropylacrylamide) hydrogels". Langmuir 23 (11): 6391–6395. doi:10.1021/la700338p. PMID 17441745.
- ↑ Roongnapa Suedee; Vatcharee Seechamnanturakit; Bhutorn Canyuk; Chitchamai Ovatlarnporn; Gary P. Martin (2006). "Temperature sensitive dopamine-imprinted (N,N-methylene-bis-acrylamide cross-linked) polymer and its potential application to the selective extraction of adrenergic drugs from urine". J. Chromatogr. A 1114 (2): 239–249. doi:10.1016/j.chroma.2006.02.033. PMID 16530207.
- ↑ Kretlow, James D.; Klouda, Leda; Mikos, Antonios G. (2007-05-30). "Injectable matrices and scaffolds for drug delivery in tissue engineering". Advanced Drug Delivery Reviews 59 (4–5): 263–273. doi:10.1016/j.addr.2007.03.013. PMID 17507111.
- ↑ Klouda, Leda; Mikos, Antonios G. (2008-01-01). "Thermoresponsive hydrogels in biomedical applications". European Journal of Pharmaceutics and Biopharmaceutics. Interactive Polymers for Pharmaceutical and Biomedical Applications 68 (1): 34–45. doi:10.1016/j.ejpb.2007.02.025. PMID 17881200.
- ↑ Klouda, Leda (2015-11-01). "Thermoresponsive hydrogels in biomedical applications: A seven-year update". European Journal of Pharmaceutics and Biopharmaceutics. Polymers for Drug Delivery Systems 97, Part B (Pt B): 338–349. doi:10.1016/j.ejpb.2015.05.017. PMID 26614556.
- ↑ Blacklow, S.; Li, J.; Freedman, B; Zeidi, Mahdi; Chen, C.; Mooney, D.J. (2019). "Bioinspired mechanically active adhesive dressings to accelerate wound closure". Science Advances 5 (7). doi:10.1126/sciadv.aaw3963. ISSN 2375-2548. PMID 31355332. Bibcode: 2019SciA....5.3963B.
- ↑ 25.0 25.1 25.2 25.3 25.4 Ward, Mark A.; Georgiou, Theoni K. (2013). "Multicompartment thermoresponsive gels: does the length of the hydrophobic side group matter?". Polymer Chemistry 4 (6): 1893. doi:10.1039/c2py21032k. https://hull-repository.worktribe.com/471429/1/Article.pdf. Retrieved 2021-06-19.
- ↑ 26.0 26.1 26.2 26.3 26.4 Ward, Mark A.; Georgiou, Theoni K. (2010-02-15). "Thermoresponsive terpolymers based on methacrylate monomers: Effect of architecture and composition". Journal of Polymer Science Part A: Polymer Chemistry 48 (4): 775–783. doi:10.1002/pola.23825. ISSN 1099-0518. Bibcode: 2010JPoSA..48..775W.
- ↑ 27.0 27.1 27.2 27.3 27.4 Ward, Mark A.; Georgiou, Theoni K. (2012). "Thermoresponsive triblock copolymers based on methacrylate monomers: effect of molecular weight and composition". Soft Matter 8 (9): 2737. doi:10.1039/c2sm06743a. Bibcode: 2012SMat....8.2737W. https://hull-repository.worktribe.com/output/466656.
- ↑ 28.0 28.1 28.2 Ward, Mark A.; Georgiou, Theoni K. (2013-07-01). "Thermoresponsive gels based on ABA triblock copolymers: Does the asymmetry matter?". Journal of Polymer Science Part A: Polymer Chemistry 51 (13): 2850–2859. doi:10.1002/pola.26674. ISSN 1099-0518. Bibcode: 2013JPoSA..51.2850W.
- ↑ Wei, Wei; Ma, Yuanzhu; Yao, Xudong; Zhou, Wenyan; Wang, Xiaozhao; Li, Chenglin; Lin, Junxin; He, Qiulin et al. (October 10, 2020). "Advanced hydrogels for the repair of cartilage defects and regeneration". Bioactive Materials 6 (4): 998–1011. doi:10.1016/j.bioactmat.2020.09.030. ISSN 2452-199X. PMID 33102942.
- ↑ Pegg, A. M. (January 2012), Baines, David; Seal, Richard, eds., "8 - The application of natural hydrocolloids to foods and beverages", Natural Food Additives, Ingredients and Flavourings, Woodhead Publishing Series in Food Science, Technology and Nutrition (Woodhead Publishing): pp. 175–196/, ISBN 978-1-84569-811-9, https://www.sciencedirect.com/science/article/pii/B9781845698119500086, retrieved 2022-10-25
- ↑ Chevalier, Olivier, "Cold gelling pastry glaze based on pectin", US patent application 2007202225, published 2007-08-30, assigned to Puratos NV, since abandoned.
- ↑ Iso, Chef. "Neutral Nappage Glaze for Fruit Tarts". https://chefiso.com/p/glaze-fruit-tart-recipe/.
- ↑ Gao, Yuan; Liu, Weifeng; Zhu, Shiping (February 8, 2017). "Polyolefin Thermoplastics for Multiple Shape and Reversible Shape Memory". ACS Applied Materials & Interfaces 9 (5): 4882–4889. doi:10.1021/acsami.6b14728. ISSN 1944-8244. PMID 28092158. https://pubs.acs.org/doi/10.1021/acsami.6b14728.
- ↑ Raduan, Norsadiah H.; Horozov, Tommy S.; Georgiou, Theoni K. (2010). ""Comb-like" non-ionic polymeric macrosurfactants". Soft Matter 6 (10): 2321. doi:10.1039/b926822g. Bibcode: 2010SMat....6.2321R. https://hull-repository.worktribe.com/output/391655.
- ↑ 35.0 35.1 Georgiou, Theoni K.; Vamvakaki, Maria; Patrickios, Costas S.; Yamasaki, Edna N.; Phylactou, Leonidas A. (2004-09-10). "Nanoscopic Cationic Methacrylate Star Homopolymers: Synthesis by Group Transfer Polymerization, Characterization and Evaluation as Transfection Reagents". Biomacromolecules 5 (6): 2221–2229. doi:10.1021/bm049755e. PMID 15530036.
- ↑ 36.0 36.1 36.2 Seuring, Jan; Agarwal, Seema (2012-11-23). "Polymers with Upper Critical Solution Temperature in Aqueous Solution". Macromolecular Rapid Communications 33 (22): 1898–1920. doi:10.1002/marc.201200433. PMID 22961764. https://onlinelibrary.wiley.com/doi/10.1002/marc.201200433.
- ↑ 37.0 37.1 Shultz, A. R.; Flory, P. J. (October 1952). "Phase Equilibria in Polymer—Solvent Systems 1,2". Journal of the American Chemical Society 74 (19): 4760–4767. doi:10.1021/ja01139a010. ISSN 0002-7863. Bibcode: 1952JAChS..74.4760S. https://www.appliedpolymertechnology.org/uploads/1/0/8/8/108867241/schultzflory_ja01139a010.pdf.link to original publication.
- ↑ "The Battery of Analytical Techniques Necessary for the Effective Characterization of Solutions of Temperature-Sensitive Polymers". Reviews and Advances in Chemistry 11 (1–2): 100–111. September 2021. doi:10.1134/S2079978021010076.
- ↑ Wohlfarth, Christian; Lide, David R. (2006). "Chapter 13: Upper Critical (UCST) and Lower Critical (LCST) Solution Temperatures of Binary Polymer Solutions". CRC Handbook of Chemistry and Physics, 87th Edition. CRC Taylor & Francis. pp. 19–34. ISBN 978-0-8493-0487-3. OCLC 644855443. https://hbcp.chemnetbase.com/faces/documents/13_06/13_06_0001.xhtml. Retrieved 2022-10-29. on Google Books.
- ↑ Hashizume, J.; Teramoto, A.; Fujita, H. (September 1981). "Phase equilibrium study of the ternary system composed of two monodisperse polystyrenes and cyclohexane". Journal of Polymer Science: Polymer Physics Edition 19 (9): 1405–1422. doi:10.1002/pol.1981.180190913. Bibcode: 1981JPoSB..19.1405H. https://onlinelibrary.wiley.com/doi/10.1002/pol.1981.180190913.
- ↑ Nakajima, Akio; Hamada, Fumiyuki; Hayashi, Seiichi (2007-03-08). "Unperturbed chain dimensions of polyethylene in theta solvents". Journal of Polymer Science Part C: Polymer Symposia 15 (1): 285–294. doi:10.1002/polc.5070150120. https://onlinelibrary.wiley.com/doi/10.1002/polc.5070150120.
- ↑ Koningsveld, R.; Staverman, A. J. (February 1968). "Liquid–liquid phase separation in multicomponent polymer solutions. III. Cloud-point curves". Journal of Polymer Science Part A-2: Polymer Physics 6 (2): 349–366. doi:10.1002/pol.1968.160060203. Bibcode: 1968JPoSB...6..349K. https://onlinelibrary.wiley.com/doi/10.1002/pol.1968.160060203.
- ↑ Fox, T. G (1962-01-01). "Properties of dilute polymer solutions III: Intrinsic viscosity/temperature relationships for conventional polymethyl methacrylate". Polymer 3: 111–128. doi:10.1016/0032-3861(62)90069-1. ISSN 0032-3861.
- ↑ Cowie, J. M. G.; McEwen, I. J. (February 1974). "Lower critical solution temperatures of polypropylene solutions". Journal of Polymer Science: Polymer Physics Edition 12 (2): 441–443. doi:10.1002/pol.1974.180120216. Bibcode: 1974JPoSB..12..441C. https://onlinelibrary.wiley.com/doi/10.1002/pol.1974.180120216.
- ↑ Pfohl, Oliver; Hino, Toshiaki; Prausnitz, John M. (1995-05-01). "Solubilities of styrene-based polymers and copolymers in common solvents". Polymer 36 (10): 2065–2073. doi:10.1016/0032-3861(95)91454-F. ISSN 0032-3861.
- ↑ Cowie, John M. G.; McEwen, Iain J. (January 1976). "Influence of microstructure on the upper and lower critical solution temperatures of poly(methylmethacrylate) solutions". Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases 72: 526–533. doi:10.1039/F19767200526. ISSN 0300-9599. https://pubs.rsc.org/en/content/articlelanding/1976/f1/f19767200526.
- ↑ Fujishige, Shouei; Kubota, K.; Ando, I. (April 1989). "Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide)". The Journal of Physical Chemistry 93 (8): 3311–3313. doi:10.1021/j100345a085. ISSN 0022-3654. https://pubs.acs.org/doi/abs/10.1021/j100345a085.
- ↑ Heskins, M.; Guillet, J. E. (1968-12-01). "Solution Properties of Poly(N-isopropylacrylamide)". Journal of Macromolecular Science: Part A - Chemistry 2 (8): 1441–1455. doi:10.1080/10601326808051910. ISSN 0022-233X.
- ↑ Kubota, Kenji; Fujishige, Shouei; Ando, Isao (January 1990). "Solution Properties of Poly(N-isopropylacrylamide) in Water". Polymer Journal 22 (1): 15–20. doi:10.1295/polymj.22.15. ISSN 1349-0540.
- ↑ Kagemoto, A.; Baba, Y. (October 25, 1971). "Study of Liquid-Liquid Phase Equilibrium by Differential Thermal Analysis". High Polymers 28 (318): 784–788; 821. doi:10.1295/koron1944.28.784. https://www.jstage.jst.go.jp/article/koron1944/28/318/28_318_784/_article/-char/ja/.
- ↑ Maeda, Yasushi; Nakamura, Tomoya; Ikeda, Isao (December 2001). "Hydration and Phase Behavior of Poly(N-vinylcaprolactam) and Poly(N-vinylpyrrolidone) in Water". Macromolecules 35 (1): 217–222. doi:10.1021/ma011034+. ISSN 0024-9297. https://pubs.acs.org/doi/10.1021/ma011034%2B.
- ↑ Amirova, Alina; Rodchenko, Serafim; Kurlykin, Mikhail; Tenkovtsev, Andrey; Krasnou, Illia; Krumme, Andres; Filippov, Alexander (March 2020). "Synthesis and Investigation of Thermo-Induced Gelation of Partially Cross-Linked Poly-2-isopropyl-2-oxazoline in Aqueous Media". Polymers 12 (3): 698. doi:10.3390/polym12030698. PMID 32245164.
- ↑ Schild, Howard G.; Tirrell, David A. (May 1, 1990). "Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions". The Journal of Physical Chemistry 94 (10): 4352–4356. doi:10.1021/j100373a088. ISSN 0022-3654. https://pubs.acs.org/doi/10.1021/j100373a088.
- ↑ Malcolm, G. N.; Rowlinson, J. S. (1957-01-01). "The thermodynamic properties of aqueous solutions of polyethylene glycol, polypropylene glycol and dioxane". Transactions of the Faraday Society 53: 921–931. doi:10.1039/TF9575300921. ISSN 0014-7672. https://pubs.rsc.org/en/content/articlelanding/1957/tf/tf9575300921.
- ↑ Saeki, Susumu; Kuwahara, Nobuhiro; Nakata, Mitsuo; Kaneko, Motozo (August 1976). "Upper and lower critical solution temperatures in poly (ethylene glycol) solutions". Polymer 17 (8): 685–689. doi:10.1016/0032-3861(76)90208-1. ISSN 0032-3861.
- ↑ Saeki, Susumu; Kuwahara, Nobuhiro; Nakata, Mitsuo; Kaneko, Motozo (1976-08-01). "Upper and lower critical solution temperatures in poly (ethylene glycol) solutions". Polymer 17 (8): 685–689. doi:10.1016/0032-3861(76)90208-1. ISSN 0032-3861.
- ↑ Van Assche, Guy; Van Mele, Bruno; Li, Ting; Nies, Erik (2011-02-22). "Adjacent UCST Phase Behavior in Aqueous Solutions of Poly(vinyl methyl ether): Detection of a Narrow Low Temperature UCST in the Lower Concentration Range". Macromolecules 44 (4): 993–998. doi:10.1021/ma102572s. ISSN 0024-9297. Bibcode: 2011MaMol..44..993V. https://pubs.acs.org/doi/10.1021/ma102572s.
- ↑ Longenecker, Ryan; Mu, Tingting; Hanna, Mark; Burke, Nicholas A. D.; Stöver, Harald D. H. (2011-11-22). "Thermally Responsive 2-Hydroxyethyl Methacrylate Polymers: Soluble–Insoluble and Soluble–Insoluble–Soluble Transitions". Macromolecules 44 (22): 8962–8971. doi:10.1021/ma201528r. ISSN 0024-9297. Bibcode: 2011MaMol..44.8962L. https://pubs.acs.org/doi/10.1021/ma201528r.
- ↑ 59.0 59.1 Kreuzer, Lucas P.; Widmann, Tobias; Geiger, Christina; Wang, Peixi; Vagias, Apostolos; Heger, Julian E.; Haese, Martin; Hildebrand, Viet et al. (2021-08-03). "Salt-Dependent Phase Transition Behavior of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Langmuir 37 (30): 9179–9191. doi:10.1021/acs.langmuir.1c01342. ISSN 0743-7463. PMID 34279952. https://pubs.acs.org/doi/10.1021/acs.langmuir.1c01342. Retrieved 2022-02-24.
- ↑ Niebuur, Bart-Jan; Puchmayr, Jonas; Herold, Christian; Kreuzer, Lucas; Hildebrand, Viet; Müller-Buschbaum, Peter; Laschewsky, André; Papadakis, Christine (2018-05-21). "Polysulfobetaines in Aqueous Solution and in Thin Film Geometry". Materials 11 (5): 850. doi:10.3390/ma11050850. ISSN 1996-1944. PMID 29883371. Bibcode: 2018Mate...11..850N.
- ↑ Mary, Pascaline; Bendejacq, Denis D.; Labeau, Marie-Pierre; Dupuis, Patrick (2007-07-01). "Reconciling Low- and High-Salt Solution Behavior of Sulfobetaine Polyzwitterions". The Journal of Physical Chemistry B 111 (27): 7767–7777. doi:10.1021/jp071995b. ISSN 1520-6106. PMID 17579398. https://pubs.acs.org/doi/10.1021/jp071995b.
- ↑ Vasantha, Vivek Arjunan; Jana, Satyasankar; Parthiban, Anbanandam; Vancso, Julius G. (2014). "Water swelling, brine soluble imidazole based zwitterionic polymers – synthesis and study of reversible UCST behaviour and gel–sol transitions". Chemical Communications 50 (1): 46–8. doi:10.1039/C3CC44407D. PMID 23925439.
- ↑ Buscall, R.; Corner, T. (1982-01-01). "The phase-separation behaviour of aqueous solutions of polyacrylic acid and its partial sodium salts in the presence of sodium chloride". European Polymer Journal 18 (11): 967–974. doi:10.1016/0014-3057(82)90084-2. ISSN 0014-3057. Bibcode: 1982EurPJ..18..967B.
- ↑ Seuring, Jan; Bayer, Frank M.; Huber, Klaus; Agarwal, Seema (2012-01-10). "Upper Critical Solution Temperature of Poly(N-acryloyl glycinamide) in Water: A Concealed Property". Macromolecules 45 (1): 374–384. doi:10.1021/ma202059t. ISSN 0024-9297. Bibcode: 2012MaMol..45..374S. https://pubs.acs.org/doi/10.1021/ma202059t.
- ↑ Liu, Fangyao; Seuring, Jan; Agarwal, Seema (2012-12-01). "Controlled radical polymerization of N -acryloylglycinamide and UCST-type phase transition of the polymers". Journal of Polymer Science Part A: Polymer Chemistry 50 (23): 4920–4928. doi:10.1002/pola.26322. Bibcode: 2012JPoSA..50.4920L. https://onlinelibrary.wiley.com/doi/10.1002/pola.26322.
- ↑ Křivánková, Nikola; Kaya, Kerem; Wijngaart, Wouter van der; Edlund, Ulrica (2023-10-04). "Copper-mediated synthesis of temperature-responsive poly(N-acryloyl glycinamide) polymers: a step towards greener and simple polymerisation". RSC Advances (The Royal Society of Chemistry) 13 (42): 29099–29108. doi:10.1039/D3RA04993K. ISSN 2046-2069. PMID 37800134. Bibcode: 2023RSCAd..1329099K.
- ↑ Shimada, Naohiko; Ino, Haruka; Maie, Kenji; Nakayama, Miki; Kano, Arihiro; Maruyama, Atsushi (2011-10-10). "Ureido-Derivatized Polymers Based on Both Poly(allylurea) and Poly( l -citrulline) Exhibit UCST-Type Phase Transition Behavior under Physiologically Relevant Conditions". Biomacromolecules 12 (10): 3418–3422. doi:10.1021/bm2010752. ISSN 1525-7797. PMID 21928793. https://pubs.acs.org/doi/10.1021/bm2010752.
- ↑ Meiswinkel, Georg; Ritter, Helmut (2013-06-25). "A new type of thermoresponsive copolymer with UCST-type transitions in water: poly(N-vinylimidazole-co-1-vinyl-2-(hydroxymethyl)imidazole)". Macromolecular Rapid Communications 34 (12): 1026–1031. doi:10.1002/marc.201300213. ISSN 1521-3927. PMID 23610054.
- ↑ Seuring, Jan; Agarwal, Seema (2012-05-08). "First Example of a Universal and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Water and Electrolyte Solution". Macromolecules 45 (9): 3910–3918. doi:10.1021/ma300355k. ISSN 0024-9297. Bibcode: 2012MaMol..45.3910S. https://pubs.acs.org/doi/10.1021/ma300355k.
- ↑ Schäfer-Soenen, H.; Moerkerke, R.; Berghmans, H.; Koningsveld, R.; Dušek, K.; Šolc, K. (1997-02-01). "Zero and Off-Zero Critical Concentrations in Systems Containing Polydisperse Polymers with Very High Molar Masses. 2. The System Water−Poly(vinyl methyl ether)". Macromolecules 30 (3): 410–416. doi:10.1021/ma960114o. ISSN 0024-9297. https://pubs.acs.org/doi/10.1021/ma960114o.
- ↑ Šolc, Karel; Dušek, Karel; Koningsveld, Ronald; Berghmans, Hugo (1995). ""Zero" and "Off-Zero" Critical Concentrations in Solutions of Polydisperse Polymers with Very High Molar Masses". Collection of Czechoslovak Chemical Communications 60 (10): 1661–1688. doi:10.1135/cccc19951661. ISSN 1212-6950. http://pikka.uochb.cas.cz/60/10/1661/.
- ↑ Afroze, F.; Nies, E.; Berghmans, H. (2000-10-01). "Phase transitions in the system poly( N-isopropylacrylamide)/water and swelling behaviour of the corresponding networks". Journal of Molecular Structure 554 (1): 55–68. doi:10.1016/S0022-2860(00)00559-7. ISSN 0022-2860. Bibcode: 2000JMoSt.554...55A. https://ui.adsabs.harvard.edu/abs/2000JMoSt.554...55A.
- ↑ Arotçaréna, Michel; Heise, Bettina; Ishaya, Sultana; Laschewsky, André (2002-04-01). "Switching the Inside and the Outside of Aggregates of Water-Soluble Block Copolymers with Double Thermoresponsivity". Journal of the American Chemical Society 124 (14): 3787–3793. doi:10.1021/ja012167d. ISSN 0002-7863. PMID 11929270. Bibcode: 2002JAChS.124.3787A. https://pubs.acs.org/doi/10.1021/ja012167d. Retrieved 2022-02-24.
- ↑ Virtanen, Janne; Arotçaréna, Michel; Heise, Bettina; Ishaya, Sultana; Laschewsky, André; Tenhu, Heikki (2002-07-01). "Dissolution and Aggregation of a Poly(NIPA- block -sulfobetaine) Copolymer in Water and Saline Aqueous Solutions". Langmuir 18 (14): 5360–5365. doi:10.1021/la0118208. ISSN 0743-7463. https://pubs.acs.org/doi/10.1021/la0118208. Retrieved 2022-02-24.
- ↑ Mertoglu, Murat; Garnier, Sébastien; Laschewsky, André; Skrabania, Katja; Storsberg, Joachim (2005-08-23). "Stimuli responsive amphiphilic block copolymers for aqueous media synthesised via reversible addition fragmentation chain transfer polymerisation (RAFT)". Polymer. Stimuli Responsive Polymers 46 (18): 7726–7740. doi:10.1016/j.polymer.2005.03.101. ISSN 0032-3861. https://www.sciencedirect.com/science/article/pii/S0032386105004891.
- ↑ Canning, Sarah L.; Neal, Thomas J.; Armes, Steven P. (2017-08-22). "pH-Responsive Schizophrenic Diblock Copolymers Prepared by Polymerization-Induced Self-Assembly". Macromolecules 50 (16): 6108–6116. doi:10.1021/acs.macromol.7b01005. ISSN 0024-9297. PMID 28867829. Bibcode: 2017MaMol..50.6108C.
- ↑ Bütün, V.; Liu, S.; Weaver, J. V. M.; Bories-Azeau, X.; Cai, Y.; Armes, S. P. (2006-01-01). "A brief review of 'schizophrenic' block copolymers". Reactive and Functional Polymers. Special Issue in Honour of John Ebdon 66 (1): 157–165. doi:10.1016/j.reactfunctpolym.2005.07.021. ISSN 1381-5148. Bibcode: 2006RFPol..66..157B. https://www.sciencedirect.com/science/article/pii/S1381514805001550.
- ↑ Papadakis, Christine M.; Müller-Buschbaum, Peter; Laschewsky, André (2019-07-30). "Switch It Inside-Out: "Schizophrenic" Behavior of All Thermoresponsive UCST–LCST Diblock Copolymers". Langmuir 35 (30): 9660–9676. doi:10.1021/acs.langmuir.9b01444. ISSN 0743-7463. PMID 31314540. https://pubs.acs.org/doi/10.1021/acs.langmuir.9b01444. Retrieved 2022-02-24.
- ↑ Kotsuchibashi, Yohei; Ebara, Mitsuhiro; Aoyagi, Takao; Narain, Ravin (2016-10-27). "Recent Advances in Dual Temperature Responsive Block Copolymers and Their Potential as Biomedical Applications". Polymers 8 (11): 380. doi:10.3390/polym8110380. ISSN 2073-4360. PMID 30974657.
- ↑ Ranka, Mikhil; Katepalli, Hari; Blankschtein, Daniel; Hatton, T. Alan (2017-11-21). "Schizophrenic Diblock-Copolymer-Functionalized Nanoparticles as Temperature-Responsive Pickering Emulsifiers". Langmuir 33 (46): 13326–13331. doi:10.1021/acs.langmuir.7b03008. ISSN 0743-7463. PMID 29064711. https://pubs.acs.org/doi/10.1021/acs.langmuir.7b03008. Retrieved 2022-02-24.
- ↑ Cummings, Chad; Murata, Hironobu; Koepsel, Richard; Russell, Alan J. (2014-03-10). "Dramatically Increased pH and Temperature Stability of Chymotrypsin Using Dual Block Polymer-Based Protein Engineering". Biomacromolecules 15 (3): 763–771. doi:10.1021/bm401575k. ISSN 1525-7797. PMID 24506329. https://pubs.acs.org/doi/10.1021/bm401575k. Retrieved 2022-02-24.
- ↑ Kreuzer, Lucas P.; Widmann, Tobias; Bießmann, Lorenz; Hohn, Nuri; Pantle, Johannes; Märkl, Raphael; Moulin, Jean-François; Hildebrand, Viet et al. (2020-04-28). "Phase Transition Kinetics of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Macromolecules 53 (8): 2841–2855. doi:10.1021/acs.macromol.0c00046. ISSN 0024-9297. Bibcode: 2020MaMol..53.2841K. https://pubs.acs.org/doi/10.1021/acs.macromol.0c00046. Retrieved 2022-02-24.
- ↑ Kreuzer, Lucas P.; Widmann, Tobias; Hohn, Nuri; Wang, Kun; Bießmann, Lorenz; Peis, Leander; Moulin, Jean-Francois; Hildebrand, Viet et al. (2019-05-14). "Swelling and Exchange Behavior of Poly(sulfobetaine)-Based Block Copolymer Thin Films". Macromolecules 52 (9): 3486–3498. doi:10.1021/acs.macromol.9b00443. ISSN 0024-9297. Bibcode: 2019MaMol..52.3486K. https://pubs.acs.org/doi/10.1021/acs.macromol.9b00443. Retrieved 2022-02-24.
- ↑ Kreuzer, Lucas P.; Widmann, Tobias; Aldosari, Nawarah; Bießmann, Lorenz; Mangiapia, Gaetano; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M. et al. (2020-10-27). "Cyclic Water Storage Behavior of Doubly Thermoresponsive Poly(sulfobetaine)-Based Diblock Copolymer Thin Films". Macromolecules 53 (20): 9108–9121. doi:10.1021/acs.macromol.0c01335. ISSN 0024-9297. Bibcode: 2020MaMol..53.9108K. https://pubs.acs.org/doi/10.1021/acs.macromol.0c01335. Retrieved 2022-02-24.
- ↑ Kreuzer, Lucas P.; Geiger, Christina; Widmann, Tobias; Wang, Peixi; Cubitt, Robert; Hildebrand, Viet; Laschewsky, André; Papadakis, Christine M. et al. (2021-08-10). "Solvation Behavior of Poly(sulfobetaine)-Based Diblock Copolymer Thin Films in Mixed Water/Methanol Vapors". Macromolecules 54 (15): 7147–7159. doi:10.1021/acs.macromol.1c01179. ISSN 0024-9297. Bibcode: 2021MaMol..54.7147K. https://pubs.acs.org/doi/10.1021/acs.macromol.1c01179. Retrieved 2022-02-24.
 |
