Chemistry:Tetrahydroxydiboron
| |
| Names | |
|---|---|
| IUPAC name
Hypoboric acid[1]
| |
| Other names
(Dihydroxyboranyl)boronic acid
Hypoboric acid Hypodiboric acid Sub-boric acid (Unterborsäure in German) 1,1,2,2-Tetrahydroxydiborane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
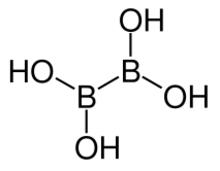
| B2H4O4 | |
| Molar mass | 89.65 g·mol−1 |
| Appearance | White powder |
| Density | 1.657 |
| Melting point | 143–148 °C (289–298 °F; 416–421 K) |
| very soluble | |
| Solubility | ethanol, DMF, DMSO, DMA |
| Structure | |
| monoclinic P21/c | |
| Thermochemistry | |
Std molar
entropy (S |
125.46 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−1410.43 kJ mol−1 |
| Hazards | |
| H302, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Diborane Diboron tetrafluoride Bis(pinacolato)diboron |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrahydroxydiboron is a chemical reagent which can be used to prepare boronic acids.[2]
Synthesis
The reaction of boron trichloride with alcohols was reported in 1931, and was used to prepare dimethoxyboron chloride, B(OCH3)2Cl.[3] Egon Wiberg and Wilhelm Ruschmann used it to prepare tetrahydroxydiboron by first introducing the boron–boron bond by reduction with sodium and then hydrolysing the resulting tetramethoxydiboron, B2(OCH3)4, to produce what they termed sub-boric acid.[4] The methanol used in this process can be recycled:
- BCl3 B(OCH3)2Cl B2(OCH3)4 B2(OH)4
Overall: 2 BCl3 + 2 Na + 4 H2O → B2(OH)4 + 2 NaCl + 4 HCl
Reactions
When heated to over 90 °C, tetrahydroxydiboron dehydrates to a polymeric boron(II) oxide. The temperature must rise to 220 °C to be totally free from water.[5]
Tetrahydroxydiboron is a reducing agent. A water solution slowly gives off hydrogen gas.[4]
References
- ↑ "Hypodiboric acid". IUPAC. https://pubchem.ncbi.nlm.nih.gov/compound/10986154#section=IUPAC-Name&fullscreen=true.
- ↑ Little, Sarah; Trice, Jane (2001). "Tetrahydroxydiboron". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289x.rn01181. ISBN 9780470842898.
- ↑ Wiberg, Egon; Sütterlin, Walther (1931). "Zur Kenntnis einiger Verbindungen vom Typus BCl3−n(OR)n. (Über alkoxyl-substituierte Borchloride)" (in German). Z. anorg. allg. Chem. 202 (1): 1–21. doi:10.1002/zaac.19312020102.
- ↑ 4.0 4.1 Wiberg, Egon; Ruschmann, Wilhelm (1937). "Über eine neue Borsäure ('Unterborsäure'︁) der Formel H4B2O4 und ihre Ester" (in de). Ber. Dtsch. Chem. Ges. A/B 70 (6): 1393–1402. doi:10.1002/cber.19370700636.
- ↑ Wartik, Thomas; Apple, Eugene F. (1955). "A New Modification of Boron Monoxide". J. Am. Chem. Soc. 77 (23): 6400–6401. doi:10.1021/ja01628a116.
 |
