Chemistry:Tetrairidium dodecacarbonyl
| |
| |
| Names | |
|---|---|
| IUPAC names
dodecacarbonyl-1κ3C,2κ3C,3κ3C,4κ3C-[Td-(13)-Δ4-closo]-tetrairidium(6 Ir—Ir)
tetrahedro-tetrakis(tricarbonyliridium)(6 Ir—Ir) | |
| Other names
iridium(0) carbonyl; iridium carbonyl; iridium dodecacarbonyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Ir4(CO)12 | |
| Molar mass | 1104.92 g/mol |
| Appearance | Canary-yellow crystals |
| Melting point | 195 °C (383 °F; 468 K) |
| Solubility | Chlorocarbons, toluene, tetrahydrofuran |
| Related compounds | |
Related compounds
|
Tetrarhodium dodecacarbonyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrairidium dodecacarbonyl is the chemical compound with the formula Ir4(CO)12. This tetrahedral cluster is the most common and most stable "binary" carbonyl of iridium. This air-stable species is only poorly soluble in organic solvents.[1][2][3] It has been used to prepare bimetallic clusters and catalysts, e.g. for the water gas shift reaction, and reforming, but these studies are of purely academic interest.
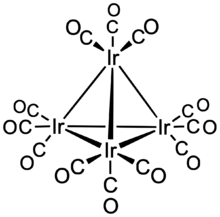
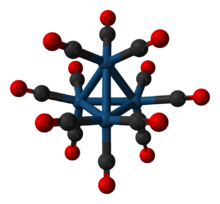
Structure
Each Ir center is octahedral, being bonded to 3 other iridium atoms and three terminal CO ligands. Ir4(CO)12 has Td symmetry with an average Ir-Ir distances of 2.693 Å.[4] The related clusters Rh4(CO)12 and Co4(CO)12 have C3v symmetry because of the presence of three bridging CO ligands in each.
Preparation
It is prepared in two steps by reductive carbonylation of hydrated iridium trichloride. The first step gives [Ir(CO)2Cl2]−.[5]
- IrCl3 + 3 CO + H2O → [Ir(CO)2Cl2]− + CO2 + 2 H+ + Cl−
- 4 [Ir(CO)2Cl2]− + 6 CO + 2 H2O → Ir4(CO)12 + 2 CO2 + 4 H+ + 8 Cl−
References
- ↑ "Tetrairidium dodecacarbonyl". https://www.webelements.com/compounds/iridium/tetrairidium_dodecacarbonyl.html.
- ↑ Uzun, Alper; Dixon, David A.; Gates, Bruce C. (10 January 2011). "Prototype Supported Metal Cluster Catalysts: Ir4 and Ir6". ChemCatChem 3 (1): 95–107. doi:10.1002/cctc.201000271. https://www.researchgate.net/publication/278129841. Retrieved 16 July 2021.
- ↑ Muetterties, E L; Burch, R R; Stolzenberg, A M (October 1982). "Molecular Features of Metal Cluster Reactions". Annual Review of Physical Chemistry 33 (1): 89–118. doi:10.1146/annurev.pc.33.100182.000513. Bibcode: 1982ARPC...33...89M. https://www.annualreviews.org/doi/abs/10.1146/annurev.pc.33.100182.000513. Retrieved 16 July 2021.
- ↑ Churchill, Melvyn Rowen; Hutchinson, John P. (1978). "Crystal Structure of tetrairidium dodecacarbonyl, Ir4(CO)12. An Unpleasant Case of Disorder". Inorganic Chemistry 17 (12): 3528–35. doi:10.1021/ic50190a040.
- ↑ Pergola, R. D.; Garlaschelli, L.; Matinengo, S. (1990). "Dodecacarbonyltetrairidium: Ir4(CO)12". Inorganic Syntheses 28: 245–247. doi:10.1002/9780470132593.ch63. ISBN 9780470132593.
 |
