Chemistry:Thiazyl fluoride

| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| NSF | |
| Molar mass | 65.07 g mol−1 |
| Appearance | colourless gas |
| Melting point | −89 °C (−128 °F; 184 K) |
| Boiling point | 0.4 °C (32.7 °F; 273.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
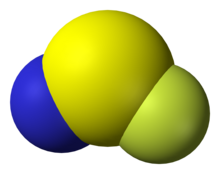
Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C.[1] Along with thiazyl trifluoride, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.
Synthesis
Thiazyl fluoride can be synthesized by various methods, such as fluorination of tetrasulfur tetranitride with silver(II) fluoride or mercuric fluoride. It can be purified by vacuum distillation.[2][3] However, because this synthetic pathway yields numerous side-products, an alternative approach is the reaction of imino(triphenyl)phosphines with sulfur tetrafluoride by cleavage of the bond to form sulfur difluoride imides and triphenyldifluorophosphorane.[4] These products readily decompose yielding thiazyl fluoride.
For synthesis on a preparative scale, the decomposition of compounds already containing the moiety is commonly used:[citation needed]
Reactivity
Reactions with electrophiles and Lewis acids
Lewis acids remove fluoride to afford thiazyl salts:[5]
- NSF + BF
3 → [NS]BF
4
Thiazyl fluoride functions as a ligand in [Re(CO)
5NSF]+
.[1] and [M(NSF)
6]{+
2} (M = Co, Ni). In all of its complexes, NSF is bound to the metal center through nitrogen.[6]
Reactions with nucleophiles
Thiazyl fluoride reacts violently with water:[7]
- NSF + 2 H
2O → SO
2 + HF + NH
3
Nucleophilic attack on thiazyl fluoride occurs at sulfur atom:.[8]
- NS–F + Nu−
→ NS–Nu + F−
Fluoride gives an adduct:
- NS–F + F−
→ NSF−
2
The halogen derivatives XNSF2 (X = F, Cl, Br, I) can be synthesized from reacting Hg(NSF)2 with X2; whereby, ClNSF2 is the most stable compound observed in this series.[9]
Oligimerization and cycloaddition
At room temperature, thiazyl fluoride undergoes cyclic trimerization via the [math]\ce{ N-S }[/math] multiple bonding:
1,3,5-trifluoro-1[math]\displaystyle{ \lambda^4 }[/math],3[math]\displaystyle{ \lambda^4 }[/math],5[math]\displaystyle{ \lambda^4 }[/math],2,4,6-trithiatriazine is the yielded cyclic trimer, where each sulfur atom remains tetravalent.
Thiazyl fluoride also reacts via exothermic cycloaddition in the presence of dienes.
Structure and bonding
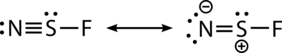
The N-S bond length is 1.448 Å, which is short, indicating multiple bonding, and can be represented by the following resonance structures:
The NSF molecule has 18 total valence electrons and is isoelectronic to sulfur dioxide. Thiazyl fluoride adopts Cs-symmetry and has been shown by isotopic substitution to be bent in the ground state.[10][11] A combination of rotational analysis with Franck-Condon calculations has been applied to study the electronic excitation from the A''[math]\displaystyle{ - }[/math]A' states, which results in the elongation of the [math]\ce{ N-S }[/math] bond by 0.11 Å and a decrease in the [math]\displaystyle{ \measuredangle }[/math]NSF by 15.3[math]\displaystyle{ ^\circ }[/math].
References
- ↑ 1.0 1.1 Oskar Glemser and Rüdiger Mews (1980). "Chemistry of Thiazyl Fluoride (NSF) and Thiazyl Trifluoride (NSF3): A Quarter Century of Sulfur-Nitrogen-Fluorine Chemistry". Angew. Chem. Int. Ed. Engl. 19 (11): 883–899. doi:10.1002/anie.198008831.
- ↑ Fischer, Gad (1974-05-01). "Vibrational assignments in the electronic spectra of thiazyl fluoride" (in en). Journal of Molecular Spectroscopy 51 (2): 208–215. doi:10.1016/0022-2852(74)90050-2. ISSN 0022-2852. https://dx.doi.org/10.1016/0022-2852%2874%2990050-2.
- ↑ Glemser, Oskar; Schröder, Hans; Haeseler, Harke (1955-01-01). "Über Schwefel-Stickstoff-Fluorverbindungen" (in de). Naturwissenschaften 42 (2): 44. doi:10.1007/BF00621536. ISSN 1432-1904. https://doi.org/10.1007/BF00621536.
- ↑ Appel, Rolf; Laßmann, Eberhard (July 1971). "Über eine neue Synthese des Thiazylfluorids, NSF" (in en). Chemische Berichte 104 (7): 2246–2249. doi:10.1002/cber.19711040727. ISSN 0009-2940. https://onlinelibrary.wiley.com/doi/10.1002/cber.19711040727.
- ↑ Mews, Rüdiger (November 1976). "Das Thionitrosylkation NS+ als Synthesereagens" (in de). Angewandte Chemie 88 (22): 757–758. doi:10.1002/ange.19760882205. https://onlinelibrary.wiley.com/doi/10.1002/ange.19760882205.
- ↑ Oskar Glemser and Rüdiger Mews (1980). "Chemistry of Thiazyl Fluoride (NSF) and Thiazyl Trifluoride (NSF3): A Quarter Century of Sulfur-Nitrogen-Fluorine Chemistry". Angew. Chem. Int. Ed. Engl. 19 (11): 883–899. doi:10.1002/anie.198008831.
- ↑ Mews, Rudiger; Keller, Klaus; Glemser, Oskar; Seppelt, K.; Thrasher, J. (2007-01-05), Shreeve, Jean'ne M., ed., "Acyclic Sulfur Nitrogen Fluorine Compounds", Inorganic Syntheses (Hoboken, NJ, USA: John Wiley & Sons, Inc.): pp. 12–17, doi:10.1002/9780470132555.ch6, ISBN 978-0-470-13255-5, https://onlinelibrary.wiley.com/doi/10.1002/9780470132555.ch6, retrieved 2022-12-15
- ↑ Cohen, B.; Hooper, T. R.; Hugill, D.; Peacock, R. D. (August 1965). "Preparation of Thiazyl Fluorides" (in en). Nature 207 (4998): 748–749. doi:10.1038/207748b0. ISSN 1476-4687. https://www.nature.com/articles/207748b0.
- ↑ Glemser, Oskar; Mews, Rüdiger; Roesky, Herbert W. (May 1969). "Darstellung und Eigenschaften von Quecksilber‐bis‐schwefeldifluoridimid, N ‐Chlor‐schwefeldifluoridimid und N ‐Brom‐schwefeldifluoridimid" (in en). Chemische Berichte 102 (5): 1523–1528. doi:10.1002/cber.19691020513. ISSN 0009-2940. https://onlinelibrary.wiley.com/doi/10.1002/cber.19691020513.
- ↑ So, Suk Ping; Richards, W. Graham (1978-01-01). "Geometries and stabilities of NSF and SNF" (in en). Journal of the Chemical Society, Faraday Transactions 2: Molecular and Chemical Physics 74: 1743–1745. doi:10.1039/F29787401743. ISSN 0300-9238. https://pubs.rsc.org/en/content/articlelanding/1978/f2/f29787401743.
- ↑ Dixon, R. N.; Duxbury, G.; Fleming, G. R.; Hugo, J. M. V. (1972-05-01). "The photoelectron spectrum of thiazyl fluoride" (in en). Chemical Physics Letters 14 (1): 60–63. doi:10.1016/0009-2614(72)87141-0. ISSN 0009-2614. https://dx.doi.org/10.1016/0009-2614%2872%2987141-0.
 |