Chemistry:Thiolactic acid
From HandWiki
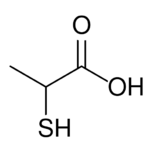
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Sulfanylpropanoic acid | |
| Other names
2-Mercaptopropanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6O2S | |
| Molar mass | 106.14 g·mol−1 |
| Density | 1.22 g/cm3 |
| Melting point | 10 °C (50 °F; 283 K) |
| Related compounds | |
Related compounds
|
lactic acid, thioglycolic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Thiolactic acid is the organosulfur compound with the formula HSCH2CO2H. The molecule contains both carboxylic acid and thiol functional groups. It is structurally related to lactic acid by the interchange of SH for OH. It is a colorless oil.
Thiolactic acid was once widely used in hair permanent waving formulations, but has been displaced by formulations based on thioglycolic acid. Instead of using the acid itself, its salts are used. It is now mainly used for depilation.[1]
See also
References
- ↑ T. Clausen (2006). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_571.pub2. ISBN 3527306730.
 |

