Chemistry:Thiophanate-methyl
From HandWiki
| |
| Names | |
|---|---|
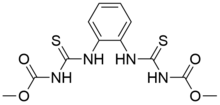
| Preferred IUPAC name
Dimethyl N,N′-[1,2-phenylenebis(azanediylcarbonothioyl)]dicarbamate | |
| Other names
Dimethyl 4,4′-(o-phenylene)bis(3-thioallophanate)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H14N4O4S2 | |
| Molar mass | 342.39 g·mol−1 |
| Appearance | white powder |
| Melting point | 172 °C (342 °F; 445 K) |
| 26.6 mg/L | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thiophanate-methyl is an organic compound with the formula C6H4(NHC(S)NH(CO)OCH3)2. The compound is a colorless or white solid, although commercial samples are generally tan-colored. It is prepared from o-phenylenediamine. It is a widely used fungicide used on tree, vine, and root crops.[1] In Europe it is applied to tomato, wine grapes, beans, wheat, and aubergine.[2]
Methods for its analysis have received considerable attention.[3][4][5] It is commonly used to treat botrytis bunch rot and gray mold caused by Botrytis cinerea strawberry in California.[6] Thiophanate-methyl acts as a fungicide via its primary metabolite carbendazim.
References
- ↑ "Thiophanate-methyl". Environmental Protection Agency. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-102001_1-Nov-04.pdf.
- ↑ European Food Safety Authority et al. (2018). "Peer review of the pesticide risk assessment of the active substance thiophanate‐methyl". EFSA Journal 16 (1): e05133. doi:10.2903/j.efsa.2018.5133. PMID 32625680.
- ↑ Mol, Hans G. J.; Plaza-Bolaños, Patricia; Zomer, Paul; De Rijk, Theo C.; Stolker, Alida A. M.; Mulder, Patrick P. J. (2008). "Toward a Generic Extraction Method for Simultaneous Determination of Pesticides, Mycotoxins, Plant Toxins, and Veterinary Drugs in Feed and Food Matrixes". Analytical Chemistry 80 (24): 9450–9459. doi:10.1021/ac801557f. PMID 19072261.
- ↑ Romero-González, R.; Garrido Frenich, A.; Martínez Vidal, J.L.; Prestes, O.D.; Grio, S.L. (2011). "Simultaneous determination of pesticides, biopesticides and mycotoxins in organic products applying a quick, easy, cheap, effective, rugged and safe extraction procedure and ultra-high performance liquid chromatography–tandem mass spectrometry". Journal of Chromatography A 1218 (11): 1477–1485. doi:10.1016/j.chroma.2011.01.034. PMID 21292276.
- ↑ Kiljanek, Tomasz; Niewiadowska, Alicja; Semeniuk, Stanisław; Gaweł, Marta; Borzęcka, Milena; Posyniak, Andrzej (2016). "Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry—Honeybee poisoning incidents". Journal of Chromatography A 1435: 100–114. doi:10.1016/j.chroma.2016.01.045. PMID 26830634.
- ↑
- Petrasch, Stefan; Knapp, Steven J.; van Kan, Jan A. L.; Blanco‐Ulate, Barbara (2019-04-04). "Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea". Molecular Plant Pathology (British Society for Plant Pathology (W-B)) 20 (6): 877–892. doi:10.1111/mpp.12794. ISSN 1464-6722. PMID 30945788.
- Cosseboom, Scott D.; Ivors, Kelly L.; Schnabel, Guido; Bryson, Patricia K.; Holmes, Gerald J. (2019). "Within-Season Shift in Fungicide Resistance Profiles of Botrytis cinerea in California Strawberry Fields". Plant Disease (American Phytopathological Society) 103 (1): 59–64. doi:10.1094/pdis-03-18-0406-re. ISSN 0191-2917. PMID 30422743.
 |

