Chemistry:Carbendazim
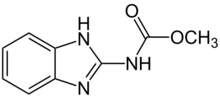
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl (1H-1,3-benzimidazol-2-yl)carbamate | |
| Other names
Mercarzole
Carbendazole | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C9H9N3O2 | |
| Molar mass | 191.187 g/mol |
| Appearance | Light gray powder |
| Density | 1.45 g/cm3 |
| Melting point | 302 to 307 °C (576 to 585 °F; 575 to 580 K) (decomposes) |
| 8 mg/L
Disintegration = 302 -305 degree Temperature of disintegration = 1.5 - 2 hrs | |
| Acidity (pKa) | 4.48 |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
Disintegration temp = 302 - 305 degree
Disintegration temp = 1.5 - 2 hrs |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carbendazim is a fungicide, a member benzimidazole fungicides. It is metabolized to benomyl, which is bioactive.[2]
The fungicide is used to control plant diseases in cereals and fruits, including citrus, bananas, strawberries, macadamia nuts, pineapples, and pomes.[3] A 4.7% solution of carbendazim hydrochloride, sold as Eertavas, is marketed as a treatment for Dutch elm disease.[4]
Other uses
It is also employed as a casting worm control agent in amenity turf situations such as golf greens, tennis courts etc. and in some countries is licensed for that use only.[5]
Safety, regulation, controversy
High doses of carbendazim cause infertility and destroy the testicles of laboratory animals.[6][7]
Maximum pesticide residue limits (MRLs) for fresh produce in the EU are between 0.1 and 0.7 mg/kg with the exception of loquat, which is 2 mg/kg.[8] The limits for more commonly consumed citrus and pome fruits are between 0.1 and 0.2 mg/kg.
Its use on macadamia plantations has proven controversial in Queensland.[4]
References
- ↑ Merck Index, 11th Edition, 1794.
- ↑ Dreikorn, Barry A.; Owen, W. John (2000). "Fungicides, Agricultural". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0621140704180509.a01. ISBN 978-0-471-48494-3.
- ↑ Wight, Andrew (14 January 2009). "Two-headed fish mystery deepens". Stock & Land. http://sl.farmonline.com.au/news/nationalrural/agribusiness-and-general/general/twoheaded-fish-mystery-deepens/1406893.aspx?storypage=0.
- ↑ 4.0 4.1 Marissa Calligeros (2009-02-02). "Fungicide maker in birth defect storm". Sydney Morning Herald. http://www.smh.com.au/news/environment/fungicide-maker-in-birth-defect-storm/2009/02/02/1233423096718.html.
- ↑ "Getting the best worm control". http://www.sherriffamenity.com/technicalupdates/Getting-The-Best-Worm-Control/90/.
- ↑ Aire, TA (August 2005). "Short-term effects of carbendazim on the gross and microscopic features of the testes of Japanese quails (Coturnix coturnix japonica).". Anatomy and Embryology 210 (1): 43–9. doi:10.1007/s00429-005-0001-0. PMID 16034611.
- ↑ "Carbendazim use banned on fruit crops". ABC. 5 February 2010. http://www.abc.net.au/rural/news/content/201002/s2811428.htm.
- ↑ "EU Pesticides Database". http://ec.europa.eu/sanco_pesticides/public/index.cfm.
External links
- International Chemical Safety Card
- Carbendazim in the Pesticide Properties DataBase (PPDB)
 |

