Chemistry:Thiophosphoric acid
| |
| Names | |
|---|---|
| Other names
Monothiophosphoric acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| H 3PO 3S | |
| Molar mass | 114.061 |
| Appearance | colorless |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
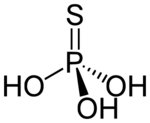
Thiophosphoric acid is an inorganic compound with the chemical formula H
3PO
3S. Structurally, it is the acid derived from phosphoric acid with one oxygen atom replaced by sulfur atom, although it cannot be prepared from phosphoric acid. It is a colorless compound that is rarely isolated in pure form, but rather as a solution. The structure of the compound has not been reported, but two tautomers are reasonable: S=P(–OH)
3 and O=P(–OH)
2(–SH).
Preparation
The compound has been prepared in a multistep process starting with the base hydrolysis of phosphorus pentasulfide to give dithiophosphate, which is isolated as its barium salt:[1]
- P
2S
5 + 6 NaOH → 2 Na
3PO
2S
2 + H
2S + 2 H
2O - 2 Na
3PO
2S
2 + 3 BaCl
2 → 2 Ba
3(PO
2S
2)
2 + 6 NaCl
In a second stage, the barium salt is decomposed with sulfuric acid, precipitating barium sulfate and liberating free dithiophosphoric acid:
- Ba
3(PO
2S
2)
2 + 3 H
2SO
4 → BaSO
4 + 2 H
3PO
2S
2
Under controlled conditions, dithiophosphoric acid hydrolyses to give the monothioderivative:
- H
3PO
2S
2 + H
2O → H
3PO
3S + H
2S
References
- ↑ R. Klement "Phosphorus" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 570, 568.
 |

