Chemistry:Thioproline
From HandWiki
| |
| Names | |
|---|---|
| Other names
(R)-1,3-Thiazolidin-3-ium-4-carboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | H-Thz-OH |
| ChEBI |
|
| ChEMBL | |
| ChemSpider |
|
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C4H7NO2S | |
| Molar mass | 133.17 g·mol−1 |
| Appearance | white solid |
| Density | 1.578 g/cm3[1] |
| Melting point | 196.5 °C (385.7 °F; 469.6 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H312, H315, H319, H332, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P280, P301+316Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P305+351+338, P317Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
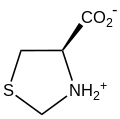
Thioproline is a nonproteinogenic amino acid with the formula (CH
2SCH
2NHCH)CO
2H, although it crystallizes as the zwitterion (CH
2SCH
2NH+
2CH)CO−
2. It consists of a 1,3-thiazolidine ring ( (CH
2SCH
2NHCH
2)) substituted with a carboxylic acid. It is synthesized by reaction of formaldehyde and cysteine.[3] It occurs in nature, but rarely.[4] It forms a coordination complex with cobalt.[5]
References
- ↑ Grant, Neil; Ward, Mark F.; Jaspars, Marcel; Harrison, William T. A. (2001). "( R )-1,3-Thiazolidin-3-ium-4-carboxylate". Acta Crystallographica Section E: Structure Reports Online 57 (8): o697–o699. doi:10.1107/S1600536801010947. Bibcode: 2001AcCrE..57O.697G.
- ↑ "L-Thioproline" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/93176#section=Safety-and-Hazards.
- ↑ Ratner, Sarah; Clarke, H. T. (1937). "The Action of Formaldehyde upon Cysteine". Journal of the American Chemical Society 59: 200–206. doi:10.1021/ja01280a050.
- ↑ Zierer, Jonas; Jackson, Matthew A.; Kastenmüller, Gabi; Mangino, Massimo; Long, Tao; Telenti, Amalio; Mohney, Robert P.; Small, Kerrin S. et al. (2018). "The fecal metabolome as a functional readout of the gut microbiome". Nature Genetics 50 (6): 790–795. doi:10.1038/s41588-018-0135-7. PMID 29808030.
- ↑ Gainsford, GJ; Jackson, WG; Sargeson, AM; Watson, AD (1980). "(4R)-Thiazolidine-4-carboxylic acid: Ligand specificity and the synthesis and X-ray structure of a cobalt(III) complex". Australian Journal of Chemistry 33 (6): 1213. doi:10.1071/CH9801213.
 |
