Chemistry:Thiostrepton

| |
| Names | |
|---|---|
| IUPAC name
N-[3-[(3-amino-3-oxoprop-1-en-2-yl)amino]-3-oxoprop-1-en-2-yl]-2-[(1R,8S,11Z,18S,25S,26R,53S,59S)-37-butan-2-yl-18-[(2S,3R)-2,3-dihydroxybutan-2-yl]-11-ethylidene-59-hydroxy-8-[(1R)-1-hydroxyethyl]-31-[(1S)-1-hydroxyethyl]-26,40,46-trimethyl-43-methylidene-6,9,16,23,28,38,41,44,47-nonaoxo-27-oxa-3,13,20,56-tetrathia-7,10,17,24,36,39,42,45,48,52,58,61,62,63,64-pentadecazanonacyclo[23.23.9.329,35.12,5.112,15.119,22.154,57.01,53.032,60]tetrahexaconta-2(64),4,12(63),19(62),21,29(61),30,32(60),33,51,54,57-dodecaen-51-yl]-1,3-thiazole-4-carboxamide
| |
| Other names
Alaninamide, Bryamycin, Thiactin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C72H85N19O18S5 | |
| Molar mass | 1664.83 g/mol |
| Appearance | White to off-white powder |
| Melting point | 246 to 256 °C (475 to 493 °F; 519 to 529 K) |
| Insoluble | |
| Solubility in other solvents | Soluble in CHCl3, CH2Cl2, dioxane, pyridine, glacial acetic acid, DMF. Practically insoluble in the lower alcohols, nonpolar organic solvents, diluted aqueous acids or bases. May be dissolved by methanolic acid or base, but with decomposition.[2] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as Streptomyces azureus and Streptomyces laurentii. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class.
History
Thiostrepton was discovered by Donovick et al. who described its antibacterial properties in 1955.[3] Dorothy Crowfoot Hodgkin solved the structure of thiostrepton in 1970.[4] Early in 1978, Bycroft and Gowland[5] proposed the biosynthesis of thiostrepton, which was still unclear until 2009. Several studies of thiopeptide biosynthesis[6][7][8][9] have been contemporarily published in 2009 and two of them (Liao et al. and Kelly et al.) included the similar biosynthesis of thiostrepton: it's ribosomally synthesized from thiostrepton biosynthetic genes (tsr genes) and posttranslational modification is needed.[citation needed]
A total synthesis of thiostrepton was completed by K.C. Nicolaou, et al. in 2004.[10][11]
Applications
Thiostrepton has been used in veterinary medicine in mastitis caused by gram-negative organisms and in dermatologic disorders. It is mostly used in complex ointments containing neomycin, nystatin, Thiostrepton and topical steroids. It is also active against gram-positive bacteria. It is notable that ointments for human usage contain neomycin, nystatin, and topical steroids, but no thiostrepton.[citation needed]
Thiostrepton was reported (in 2008) to exhibit activity against breast cancer cells through targeting the transcription factor forkhead box M1 (FOXM1),[12] also in 2011.[13] It has also been shown to circumvent acquired cisplatin resistance in breast cancer cells under in vitro conditions.[14]
Thiostrepton was reported to be a covalent inhibitor of the mitochondrial enzyme Peroxiredoxin 3 (PRDX3).[15][16] PRDX3 is a mitochondrial localized peroxidase that converts hydrogen peroxide to water. Thiostrepton inactivates the active site cysteines of PRDX3, inactivating it's peroxidase activity, leading to cytoxic levels of hydrogen peroxide incompatible with tumor cell growth.[15][16] Thiostrepton is the active pharmaceutical ingredient in RSO-021. RSO-021 is currently being tested in the MITOPE phase1/2 clinical trial in patients with malignant pleural effusion arising from mesothelioma or other solid tumors. RSO-021 has met its primary end point of safety and tolerability in phase 1 testing.
Thiostrepton is used in molecular biology as a reagent for both positive and negative selection of genes involved in nucleotide metabolism.[citation needed]
Thiostrepton has also shown promise in treating osteoporosis in animal models because it can inhibit unusual osteoclast precursor cells.[17]
The antibiotic thiostrepton was identified as an insulin resistance reversal agent. Subsequent validation in ex vivo insulin-resistant mouse muscle and palmitate-induced insulin-resistant myotubes demonstrated potent insulin action restoration, possibly via upregulation of glycolysis due to attenuation of mitochondrial oxidative phosphorylation by thiostrepton.[18]
Biosynthesis
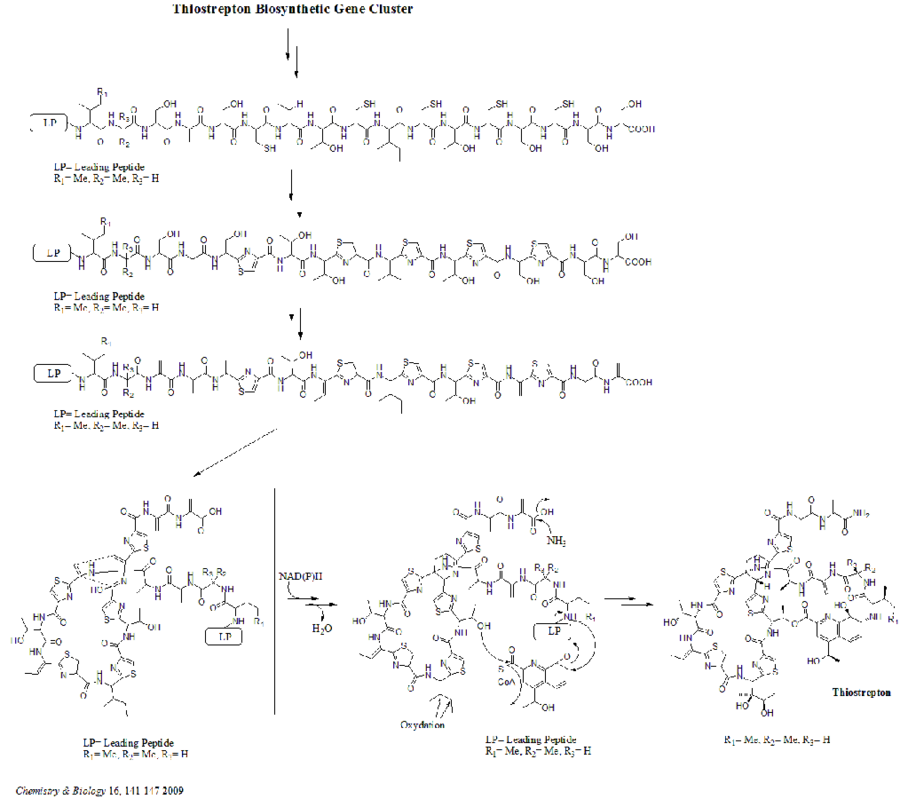
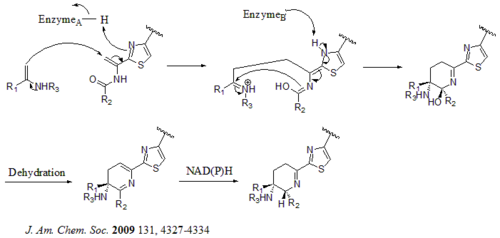
There are total 21 genes (tsrA~tsrU) in the biosynthetic gene cluster. The precursor of thiostrepton contains 58 amino acids in the peptide chain, which includes 41-aa leader peptide (LP) and 17-aa structural peptide (IASASCTTCICTCSCSS). Once the precursor is synthesized, cyclodehydratase tsrO and dehydrogenase tsrM catalyze the formation of thiazole or thiazoline from every cysteine residues in the peptide chain. After thiazole/thiazoline formation, dehydratases tsrJ, K and S then convert all the serine residues into dehydroalanines. A hetero Diels-Alder cyclization of the central dehydropiperidine (at S5, C13, and S14) has been suggested by Bycroft back to 1978 and been employed in the chemical synthesis of this core structure by Nicolaou et al. in 2005. An alternative mechanism of the dehydropiperidine formation has also been suggested by Kelly et al. in 2009. Nevertheless, based on experimental evidence, tsrN and L are suggested to be responsible for the hetero Diels-Alder cyclization. The quinaldic acid moiety is suggested to be synthesized by the nine genes tsrFAEBDUPQI from tryptophan and then results in the closure of quinaldic acid macrocycle. At last, tsrR serves as a candidate for the oxidation of the Ile residue to afford thiostrepton.[citation needed]
Alternative mechanism for the formation of the dehydropiperidine core
Total synthesis
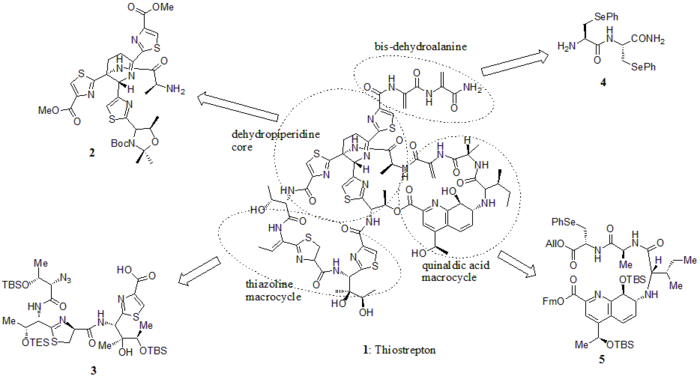
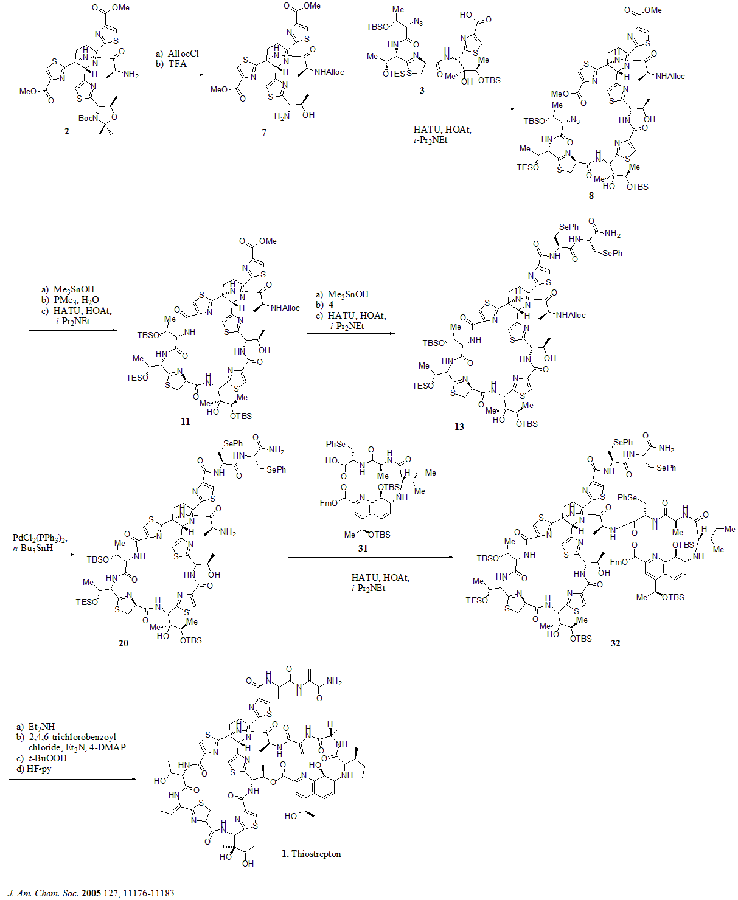
In 2005, Nicolaou et al. published the total synthesis of thiostrepton. At first, they constructed the key building blocks of thiostrepton (1): dehydropiperidine core (2), thiazoline macrocycle (3), bis-dehydroalanine tail (4), and quinaldic acid macrocycle (5). Then they assembled the building blocks sequentially as shown in the synthetic scheme (compound numbers are from the reference).
Building blocks
Synthetic scheme
References
- ↑ Merck Index, 11th Edition, 9295.
- ↑ Thiostrepton product page at Fermentek
- ↑ "Thiostrepton, a new antibiotic. I. In vitro studies". Antibiot Annu 3: 554–9. 1955. PMID 13355325.
- ↑ "The Structure of Thiostrepton". Nature 225 (5229): 223–235. 1970. doi:10.1038/225233a0. PMID 5409975. Bibcode: 1970Natur.225..233A.
- ↑ Bycroft, Barrie W.; Gowland, Maxim S. (1978). "The structures of the highly modified peptide antibiotics micrococcin P1 and P2". Journal of the Chemical Society, Chemical Communications (6): 256. doi:10.1039/c39780000256. ISSN 0022-4936.
- ↑ "Thirteen posttranslational modifications convert a 14-residue peptide into the antibiotic thiocillin". Proc. Natl. Acad. Sci. U.S.A. 106 (8): 2549–53. 2009. doi:10.1073/pnas.0900008106. PMID 19196969. Bibcode: 2009PNAS..106.2549W.
- ↑ "Ribosomally synthesized thiopeptide antibiotics targeting elongation factor Tu". J. Am. Chem. Soc. 131 (16): 5946–55. 2009. doi:10.1021/ja900488a. PMID 19338336.
- ↑ "Thiopeptide biosynthesis featuring ribosomally synthesized precursor peptides and conserved posttranslational modifications". Chem. Biol. 16 (2): 141–7. 2009. doi:10.1016/j.chembiol.2009.01.007. PMID 19246004.
- ↑ "Thiostrepton biosynthesis: prototype for a new family of bacteriocins". J. Am. Chem. Soc. 131 (12): 4327–34. 2009. doi:10.1021/ja807890a. PMID 19265401.
- ↑ Nicolaou, K. C.; Zak, Mark; Safina, Brian S.; Estrada, Anthony A.; Lee, Sang Hyup; Nevalainen, Marta (2005). "Total Synthesis of Thiostrepton. Assembly of Key Building Blocks and Completion of the Synthesis". Journal of the American Chemical Society 127 (31): 11176–11183. doi:10.1021/ja052934z. ISSN 0002-7863. PMID 16076225.
- ↑ "Total synthesis of thiostrepton. Retrosynthetic analysis and construction of key building blocks". J. Am. Chem. Soc. 127 (31): 11159–75. 2005. doi:10.1021/ja0529337. PMID 16076224.
- ↑ "Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression". Mol. Cancer Ther. 7 (7): 2022–32. July 2008. doi:10.1158/1535-7163.MCT-08-0188. PMID 18645012.
- ↑ http://www.news-medical.net/news/20110822/Scientists-reveal-how-thiostrepton-blocks-FOXM1-protein-prevents-breast-cancer-development.aspx Scientists reveal how thiostrepton blocks FOXM1 protein, prevents breast cancer development. 2011
- ↑ Kwok JM; Peck B; Monteiro LJ; Schwenen HD; Millour J; Coombes RC; Myatt SS; Lam EW. (January 2010). "FOXM1 confers acquired cisplatin resistance in breast cancer cells". Molecular Cancer Research 8 (1): 24–34. doi:10.1158/1541-7786.MCR-09-0432. PMID 20068070.
- ↑ 15.0 15.1 Cunniff, Brian; Newick, Kheng; Nelson, Kimberly J.; Wozniak, Alexandra N.; Beuschel, Stacie; Leavitt, Bruce; Bhave, Anant; Butnor, Kelly et al. (2015-05-26). "Disabling Mitochondrial Peroxide Metabolism via Combinatorial Targeting of Peroxiredoxin 3 as an Effective Therapeutic Approach for Malignant Mesothelioma". PLOS ONE 10 (5): e0127310. doi:10.1371/journal.pone.0127310. ISSN 1932-6203. PMID 26011724. Bibcode: 2015PLoSO..1027310C.
- ↑ 16.0 16.1 Nelson, Kimberly J.; Messier, Terri; Milczarek, Stephanie; Saaman, Alexis; Beuschel, Stacie; Gandhi, Uma; Heintz, Nicholas; Smalley, Terrence L. et al. (2021-01-20). "Unique Cellular and Biochemical Features of Human Mitochondrial Peroxiredoxin 3 Establish the Molecular Basis for Its Specific Reaction with Thiostrepton". Antioxidants 10 (2): 150. doi:10.3390/antiox10020150. ISSN 2076-3921. PMID 33498547.
- ↑ Masaru Ishii (February 2023). The Culprit Behind Osteoporosis - Medical Frontiers. NHK TV.
- ↑ Masson, S. W., Madsen, S., Cooke, K. C., Potter, M., Vegas, A. D., Carroll, L., ... & James, D. E. (2023). Leveraging genetic diversity to identify small molecules that reverse mouse skeletal muscle insulin resistance. Elife, 12, RP86961 https://doi.org/10.7554/eLife.86961; bioRxiv doi:10.1101/2023.03.01.530673
 |