Chemistry:Transition metal complexes of pyridine-N-oxides
Transition metal complexes of pyridine-N-oxides encompass coordination complexes that contain pyridine-N-oxides as ligands. Particularly common are the octahedral homoleptic complexes of the type [M(ONC
5H
5)
6]2+
where M = Mn(II), Fe(II), Co(II), Ni(II).[2] Many variations of pyridine N-oxide are known, such as the dioxides of 2,2'- and 4,4'-2,2'-bipyridine.[3] Complexes derived from the trioxide of terpyridine have been crystallized as well.[4]
Structure and bonding
Pyridine-N-oxides bind to metals through the oxygen. According to X-ray crystallography, the M-O-N angle is approximately 130° in many of these complexes. As reflected by the pKa of 0.79 for C
5H
5NOH+
, pyridine N-oxides are weakly basic ligands. Their complexes are generally high spin, hence they are kinetically labile.
Applications
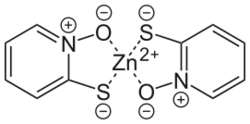
Zinc pyrithione is a coordination complex of a sulfur-substituted pyridine-N-oxide. This zinc complex has useful fungistatic and bacteriostatic properties..[5]
References
- ↑ Van Ingen Schenau, A. D.; Verschoor, C. G.; Romers, C. (1974). "The Crystal and Molecular Structure of Hexakis(pyridine-N-oxide)nickel(II) Bis(tetrafluoroborate)". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 30 (7): 1686–1694. doi:10.1107/S0567740874005632.
- ↑ Carlin, Richard L.; De Jongh, L. J. (1986). "Structural and Magnetic Properties of Transition Metal complexes of Pyridine N-Oxide". Chemical Reviews 86 (4): 659–680. doi:10.1021/cr00074a001.
- ↑ Jia, Junhua; Hubberstey, Peter; Champness, Neil R.; Schröder, Martin (2009). "Supramolecular Chemistry of 4,4′-Bipyridine-N,N′-dioxide in Transition Metal Complexes: A Rich Diversity of Co-ordinate, Hydrogen-Bond and Aromatic Stacking Interactions". Molecular Networks. Structure and Bonding. 132. pp. 135–161. doi:10.1007/430_2008_9. ISBN 978-3-642-01366-9.
- ↑ Amoroso, Angelo J.; Burrows, Miles W.; Dickinson, Anthony A.; Jones, Cameron; Willock, David J.; Wong, Wing-Tak (2001). "Geometrical preferences of complexes of terpyridine N-oxide ligands: Synthesis and crystal structures of nickel(II) with terpyridine 1,1′,1″-trioxide, terpyridine 1,1″-dioxide and terpyridine 1-oxide". Journal of the Chemical Society, Dalton Transactions (3): 225–227. doi:10.1039/b008993l.
- ↑ Barnett, B. L.; Kretschmar, H. C.; Hartman, F. A. (1977). "Structural characterization of bis(N-oxopyridine-2-thionato)zinc(II)". Inorg. Chem. 16 (8): 1834–8. doi:10.1021/ic50174a002.
 |


