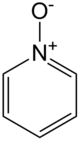
Chemistry:Pyridine-N-oxide
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1λ5-Pyridin-1-one | |||
| Other names
Pyridine-1-oxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C5H5NO | |||
| Molar mass | 95.101 g·mol−1 | ||
| Appearance | Colourless solid | ||
| Melting point | 65 to 66 °C (149 to 151 °F; 338 to 339 K) | ||
| Boiling point | 270 °C (518 °F; 543 K) | ||
| high | |||
| Acidity (pKa) | 0.8 (of conjugate acid) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Pyridine-N-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peroxyacids as the oxidising agent. The compound is used infrequently as an oxidizing reagent in organic synthesis.[1]
Structure
The structure of pyridine-N-oxide is very similar to that of pyridine with respect to the parameters for the ring. The molecule is planar. The N-O distance is 1.34 Å. The C-N-C angle is 124°, 7° wider than in pyridine.[2]
Synthesis
The oxidation of pyridine can be achieved with a number of peracids including peracetic acid and perbenzoic acid.[3] Oxidation can also be effected by a modified Dakin reaction using a urea-hydrogen peroxide complex,[4] and sodium perborate[5] or, using methylrhenium trioxide (CH3ReO3) as catalyst, with sodium percarbonate.[6]
Reactions
Pyridine N-oxide is five orders of magnitude less basic than pyridine: the pKa of protonated pyridine-N-oxide is 0.8.[7] Protonated derivatives are isolable, e.g., [C5H5NOH]Cl.[3] Further demonstrating its (feeble) basicity, pyridine-N-oxide also serves as a ligand in coordination chemistry. A host of transition metal complexes of pyridine-N-oxides are known.
Treatment of the pyridine-N-oxide with phosphorus oxychloride gives 4- and 2-chloropyridines.[8]
Related pyridine-N-oxides
The N-oxides of various pyridines are precursors to useful drugs:[9]
- Nicotinic acid N-oxide, derived from nicotinic acid is a precursor to niflumic acid and pranoprofen.
- 2,3,5-trimethylpyridine N-oxide is a precursor to the drug omeprazole
- 2-chloropyridine N-oxide is a precursor to the fungicide zinc pyrithione
Safety
The compound is a skin irritant.[1]
Further reading
- discovery of pyridine-N-oxide: Meisenheimer, Jakob (1926). "Über Pyridin-, Chinolin- und Isochinolin-N-oxyd" (in German). Ber. Dtsch. Chem. Ges. 59 (8): 1848–1853. doi:10.1002/cber.19260590828.
- Synthesis of N-oxides from substituted pyridines: Youssif, Shaker (2001). "Recent trends in the chemistry of pyridine N-oxides". Arkivoc 2001: 242–268. doi:10.3998/ark.5550190.0002.116. http://www.arkat-usa.org/get-file/19897/.
References
- ↑ 1.0 1.1 Kilényi, S. Nicholas; Mousseau, James J. (20 September 2015). "Pyridine N-Oxide". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. pp. 1–6. doi:10.1002/047084289X.rp283.pub2. ISBN 9780470842898.
- ↑ Ülkü, D.; Huddle, B. P.; Morrow, J. C. (1971). "The Crystal Structure of Pyridine 1-oxide". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 27 (2): 432–436. doi:10.1107/S0567740871002334.
- ↑ 3.0 3.1 Mosher, H. S.; Turner, L.; Carlsmith, A. (1953). "Pyridine-N-oxide". Org. Synth. 33: 79. doi:10.15227/orgsyn.033.0079.
- ↑ Varma, Rajender S.; Naicker, Kannan P. (1999). "The Urea−Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles". Org. Lett. 1 (2): 189–192. doi:10.1021/ol990522n.
- ↑ McKillop, Alexander; Kemp, Duncan (1989). "Further functional group oxidations using sodium perborate". Tetrahedron 45 (11): 3299–3306. doi:10.1016/S0040-4020(01)81008-5.
- ↑ Jain, Suman L.; Joseph, Jomy K.; Sain, Bir (2006). "Rhenium-Catalyzed Highly Efficient Oxidations of Tertiary Nitrogen Compounds to N-Oxides Using Sodium Percarbonate as Oxygen Source". Synlett 2006 (16): 2661–2663. doi:10.1055/s-2006-951487.
- ↑ Chmurzyński, L. (1996). "Studies on correlations of acid-base properties of substituted pyridine N-oxides in solutions. Part 1. Correlations of the p Ka values in non-aqueous solvents and water". Analytica Chimica Acta 321 (2–3): 237–244. doi:10.1016/0003-2670(95)00594-3.
- ↑ Scriven, E. F. V. (1984). "Pyridines and their Benzo Derivatives: (ii) Reactivity at Ring Atoms". in Katritzky, Alan R.; Rees, Charles Wayne; Meth-Cohn, Otto. Comprehensive Heterocyclic Chemistry: The Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds. 2. Pergamon Press. pp. 165–314. doi:10.1016/B978-008096519-2.00027-8. ISBN 9780080307015.
- ↑ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
 |