Chemistry:Transition metal complexes of thiocyanate
Transition metal complexes of thiocyanate describes coordination complexes containing one or more thiocyanate (SCN-) ligands. The topic also includes transition metal complexes of isothiocyanate. These complexes have few applications but played significant role in the development of coordination chemistry.
Structure and bonding
Hard metal cationss, as classified by HSAB theory, tend to form N-bonded complexes (isothiocyanates), whereas class B or soft metal cations tend to form S-bonded thiocyanate complexes. For the isothiocyanates, the M-N-C angle is usually close to 180°. For the thiocyanates, the M-S-C angle is usually close to 100°.
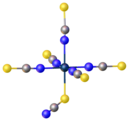
Crystal structure of [NiII(NCS)6]4-, a homoleptic complex of six isothiocyanate ligands. Color code: blue = N, yellow = S.
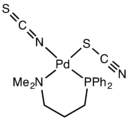
Structure of Pd(Me2N(CH2)3PPh2)(SCN)(NCS) illustrating linkage isomerism of the SCN- ligand..[1]
Crystal structure]] of [ReIV(NCS)5(SCN)]2-.[2] Color code: blue = N, yellow = S.
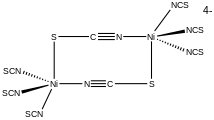
Structure of the dinuclear complex [NiII2(SCN)8]4- with a bridging SCN- ligand.
Homoleptic complexes
Most homoleptic complexes of NCS- feature isothiocyanate ligands (N-bonded). All first-row metals bind thiocyanate in this way.[3] Octahedral complexes [M(NCS)6]z- include M = Ti(III), Cr(III), Mn(II), Fe(III), Ni(II), Mo(III), Tc(IV), and Ru(III). Four-coordinated tetrakis(isothiocyanate) complexes would be tetrahedral since isothiocyanate is a weak-field ligand. Two examples are the deep blue [Co(NCS)4]2- and the green [Ni(NCS)4]2-.[4]
Few homoleptic complexes of NCS- feature thiocyanate ligands (S-bonded). Octahedral complexes include [M(SCN)6]3- (M = Rh[5] and Ir[6]) and [Pt(SCN)6]2-. Square planar complexes include [M(SCN)4]z- (M = Pd(II), Pt(II),[7] and Au(III)). Colorless [Hg(SCN)4]2- is tetrahedral.
Some octahedral isothiocyanate complexes exist in two oxidation states. Orange [Os(NCS)6]3- can be oxidized to violet [Os(NCS)6]2-. The Os-N distances in both derivatives are almost identical at 200 picometers.[8]
Linkage isomerism
Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen.[9] Thiocyanate can bind metals at either sulfur or nitrogen — it is an ambidentate ligand. Other factors, e.g. kinetics and solubility, sometimes influence the observed isomer. For example, [Co(NH3)5(NCS)]+ is the thermodynamic isomer, but [Co(NH3)5(SCN)]2+ forms as the kinetic product of the reaction of thiocyanate salts with [Co(NH3)5(H2O)]3+.[10]
- [Co(NH
3)
5(H
2O)]3+ + SCN−
→ [Co(NH
3)
5(SCN)]2+ + H
2O - [Co(NH
3)
5(SCN)]2+ → [Co(NH
3)
5(NCS)]2+
Some complexes of SCN- feature both but only thiocyanate and isothiocyanate ligands. Examples are found for heavy metals in the middle of the d-period: Ir(III),[11] and Re(IV).[2]
SCN-bridged complexes
As a ligand, [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). One example of an SCN-bridged complex is [Ni2(SCN)8]4-.[4]
Mixed ligand complexes
This article focuses on homoleptic complexes, which are simpler to describe and analyze. Most complexes of SCN-, however are mixed ligand species. Mentioned above is one example, [Co(NH3)5(NCS)]2+. Another example is [OsCl2(SCN)2(NCS)2]2-.[12] Reinecke's salt, a precipitating agent, is a derivative of [Cr(NCS)4(NH3)2]-.
References
- ↑ Palenik, Gus J.; Clark, George Raymond (1970). "Crystal and Molecular Structure of Isothiocyanatothiocyanato-(1-diphenylphosphino-3-dimethylaminopropane)palladium(II)". Inorganic Chemistry 9 (12): 2754–2760. doi:10.1021/ic50094a028. ISSN 0020-1669.
- ↑ 2.0 2.1 González, Ricardo; Barboza, Natalia; Chiozzone, Raúl; Kremer, Carlos; Armentano, Donatella; De Munno, Giovanni; Faus, Juan (2008). "Linkage Isomerism in the Metal Complex Hexa(thiocyanato)rhenate(IV): Synthesis and Crystal Structure of (NBu4)2[Re(NCS)6] and [Zn(NO3)(Me2phen)2]2[Re(NCS)5(SCN)]". Inorganica Chimica Acta 361 (9–10): 2715–2720. doi:10.1016/j.ica.2008.01.017.
- ↑ Shurdha, Endrit; Moore, Curtis E.; Rheingold, Arnold L.; Lapidus, Saul H.; Stephens, Peter W.; Arif, Atta M.; Miller, Joel S. (2013). "First Row Transition Metal(II) Thiocyanate Complexes, and Formation of 1-, 2-, and 3-Dimensional Extended Network Structures of M(NCS)2(Solvent)2 (M = Cr, Mn, Co) Composition". Inorganic Chemistry 52 (18): 10583–10594. doi:10.1021/ic401558f. PMID 23981238.
- ↑ 4.0 4.1 Larue, Bruno; Tran, Lan-Tâm; Luneau, Dominique; Reber, Christian (2003). "Crystal Structures, Magnetic Properties, and Absorption Spectra of Nickel(II) Thiocyanato Complexes: A Comparison of Different Coordination Geometries". Canadian Journal of Chemistry 81 (11): 1168–1179. doi:10.1139/v03-114.
- ↑ Vogt, J.‐U.; Haeckel, O.; Preetz, W. (1995). "Darstellung und Kristallstruktur von Tetraphenylphosphonium‐Hexathiocyanatorhodat(III), [P(C6H5)4]3[Rh(SCN)6]". Zeitschrift für Anorganische und Allgemeine Chemie 621 (6): 1033–1036. doi:10.1002/zaac.19956210623.
- ↑ Rohde, J.-U.; Preetz, W. (1998). "Kristallstruktur von (Me4N)3[Ir(SCN)6], Schwingungsspektrum und Normalkoordinatenanalyse". Zeitschrift für Anorganische und Allgemeine Chemie 624 (8): 1319–1323. doi:10.1002/(SICI)1521-3749(199808)624:8<1319::AID-ZAAC1319>3.0.CO;2-Q.
- ↑ Rohde, J.-U.; Malottki, B. von; Preetz, W. (2000). "Kristallstrukturen, Spektroskopische Charakterisierung und Normalkoordinatenanalyse von (n-Bu4N)2[M(ECN)4] (M = Pd, Pt; E = S, Se)". Zeitschrift für Anorganische und Allgemeine Chemie 626 (4): 905–910. doi:10.1002/(SICI)1521-3749(200004)626:4<905::AID-ZAAC905>3.3.CO;2-Q.
- ↑ Stähler, O.; Preetz, W. (2001). "Kristallstrukturen, Schwingungsspektren und Normalkoordinatenanalyse von (n-Bu4N)2[Os(NCS)6] und (n-Bu4N)3[Os(NCS)6]". Zeitschrift für Anorganische und Allgemeine Chemie 627 (4): 615–619. doi:10.1002/1521-3749(200104)627:4<615::AID-ZAAC615>3.0.CO;2-4.
- ↑ Burmeister, J. (1990). "Ambidentate Ligands, the Schizophrenics of Coordination Chemistry". Coordination Chemistry Reviews 105: 77–133. doi:10.1016/0010-8545(90)80019-P.
- ↑ Buckingham, David A.; Creaser, I. I.; Sargeson, A. M. (1970). "Mechanism of Base Hydrolysis for CoIII(NH3)5X2+ Ions. Hydrolysis and Rearrangement for the Sulfur-Bonded Co(NH3)5SCN2+ Ion". Inorganic Chemistry 9 (3): 655–661. doi:10.1021/ic50085a044.
- ↑ Semrau, M.; Preetz, W. (1996). "Darstellung und Kristallstruktur von (n‐Bu4N)3[Ir(NCS)(SCN)5]". Zeitschrift für Anorganische und Allgemeine Chemie 622 (11): 1953–1956. doi:10.1002/zaac.19966221123.
- ↑ Semrau, M.; Preetz, W. (1996). "Darstellung und Kristallstruktur von trans ‐(Ph4As)2[OsCl2(NCS)2(SCN)2], Schwingungsspektren und Normalkoordinatenanalyse". Zeitschrift für Anorganische und Allgemeine Chemie 622 (9): 1537–1541. doi:10.1002/zaac.19966220916.
 |