Chemistry:Trimethylenemethane
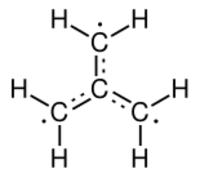 Trimethylenemethane, average of three configurations. Formally, the radial bonds have valency 4/3. Each terminal carbon has 2/3 of an unfilled valence bond.
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylidenepropane-1,3-diyl | |
| Other names
Trimethylenemethane biradical; Trimethylenemethane diradical
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4H6 | |
| Molar mass | 54.092 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethylenemethane (often abbreviated TMM) is a chemical compound with formula C4H6. It is a neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, it can be viewed as an isobutylene molecule C4H8 with two hydrogen atoms removed from the terminal methyl groups.
Structure
The electronic structure of trimethylenemethane was discussed in 1948.[1][2] It is a neutral four-carbon molecule containing four pi molecular orbitals. When trapped in a solid matrix at about 90 K (−183 °C), the six hydrogen atoms of the molecule are equivalent. Thus, it can be described either as zwitterion, or as the simplest conjugated hydrocarbon that cannot be given a Kekulé structure. It can be described as the superposition of three states:
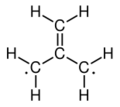
|
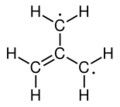
|
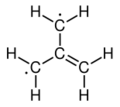
|
It has a triplet ground state (3A2′/3B2), and is therefore a diradical in the stricter sense of the term.[3] Calculations predict a planar molecule with three-fold rotational symmetry, with approximate bond lengths 1.40 Å (C–C) and 1.08 Å (C–H). The H–C–H angle in each methylene is about 121°.[1]
Of the three singlet excited states, the first one, 11A1 (1.17 eV above ground), is a closed shell diradical with flat geometry and fully degenerate threefold (D3h) symmetry. The second one, 11B2 (also at 1.17 eV), is an open-shell radical with a D3h-symmetric equilibrium between three equal geometries; each has a longer C–C bond (1.48 Å) and two shorter ones (1.38 Å), and is flat and bilaterally symmetric except that the longer methylene is twisted 79° out of the plane (C2 symmetry). The third singlet state, 21A1/1A1′ (3.88 eV), is also a D3h-symmetric equilibrium of three geometries; each is planar with one shorter C–C bond and two longer ones (C2ν symmetry).[1]
The next higher energy states are degenerate triplets, 13A1 and 23B2 (4.61 eV), with one excited electron; and a quintet state, 5B2 (7.17 eV), with the p orbitals occupied by single electrons and D3h symmetry.[1]
Preparation
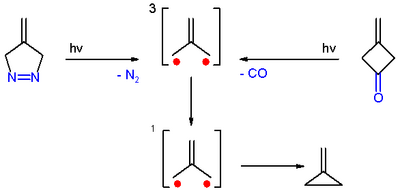
Trimethylenemethane was first obtained from photolysis of the diazo compound 4-methylene-Δ1-pyrazoline with expulsion of nitrogen, in a frozen dilute glassy solution at −196 °C (77 K).[3]
It was also obtained from photolysis of 3-methylenecyclobutanone, both in cold solution and in the form of a single crystal, with expulsion of carbon monoxide. In both cases, trimethylenemethane was detected by electron spin resonance spectroscopy.[3]
Trimethylenemethane has been obtained also by treating potassium with 2-iodomethyl-3-iodopropene and isobutylene diiodide (IH2C)2C=CH2 in the gas phase. However the product quickly dimerizes to yield 1,4-dimethylenecyclohexane, and also 2-methylpropene by abstracting two hydrogen atoms from other molecules (hydrocarbon or potassium hydride).[4]
Organometallic chemistry
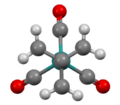
A number of organometallic complexes have been prepared, starting with Fe(C4H6)(CO)3, which was obtained by the ring-opening of methylenecyclopropane with diiron nonacarbonyl (Fe2(CO)9).[3] The same complex was prepared by the salt metathesis reaction of disodium tetracarbonylferrate (Na2Fe(CO)4) with 1,1-bis(chloromethyl)ethylene (H2C=C(CH2Cl)2).[5] Related reactions give M(TMM)(CO)4 (M = Cr, Mo). The reaction leading to (TMM)Mo(CO)4 also gives Mo(C8H12)(CO)3 containing a dimerized TMM ligand.[5]
TMM complexes have been examine for their potential in organic synthesis, specifically in the trimethylenemethane cycloaddition reaction with only modest success. One example is a palladium-catalyzed [3+2]cycloaddition of trimethylenemethane.[6]
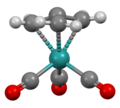
Structure of Ru(trimethylenemethane)(CO)3, viewed down C3 axis.[7]
References
- ↑ 1.0 1.1 1.2 1.3 Slipchenko Lyudmila V., Krylov Anna I. (2003). "Electronic structure of the trimethylenemethane diradical in its ground and electronically excited states: Bonding, equilibrium geometries, and Vibrational frequencies". Journal of Chemical Physics 118 (15): 6874–6883. doi:10.1063/1.1561052. Bibcode: 2003JChPh.118.6874S.
- ↑ C. A. Coulson (1948), Journal de Chimie Physique et de Physico-Chimie Biologique, volume 45, page 243. Cited by Slipchenko and Krylov (2003)
- ↑ 3.0 3.1 3.2 3.3 Paul Dowd (1972). "Trimethylenemethane". Accounts of Chemical Research 5 (7): 242–248doi=10.1021/ar50055a003. doi:10.1021/ar50055a003.
- ↑ Skell Philip S., Doerr Robert G. (1967). "Trimethylenemethane". Journal of the American Chemical Society 89 (18): 4688–4692. doi:10.1021/ja00994a020.
- ↑ 5.0 5.1 J. S. Ward; R. Pettit (1970). "Trimethylenemethane complexes of iron, molybdenum, and chromium". Journal of the Chemical Society D (21): 1419–1420. doi:10.1039/C29700001419.
- ↑ Trost Barry M (1979). "New conjunctive reagents. 2-Acetoxymethyl-3-allyltrimethylsilane for methylenecyclopentane annulations catalyzed by palladium(0)". Journal of the American Chemical Society 101 (21): 6429–6432. doi:10.1021/ja00515a046.
- ↑ Herberich, G. E.; Spaniol, T. P. (1993). "Trimethylenemethane Complexes of Ruthenium, Osmium and Rhodium Via the Compound CH2=C(CH2SnMe3)2". Journal of the Chemical Society, Dalton Transactions (16): 2471–2476. doi:10.1039/DT9930002471.
 |