Chemistry:Trimethylplatinum iodide
| |
| Names | |
|---|---|
| Other names
Iodotrimethylplatinum(IV)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C12H36I4Pt4 | |
| Molar mass | 1468.374 g·mol−1 |
| Appearance | white solid |
| Melting point | 190-195 °C |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H228, H302, H312, H315, H319, H332, H413 | |
| P210, P240, P241, P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P370+378, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
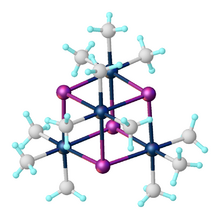
Trimethylplatinum iodide is the organoplatinum complex with the formula [(CH3)3PtI]4. It is a white, air-stable solid that was one of the first σ-alkyl metal complexes reported.[1] It arises from the reaction of potassium hexachloroplatinate with methylmagnesium iodide.[2] The complex exists as a tetramer: a cubane-type cluster with four octahedral Pt(IV) centers linked by four iodides as triply bridging ligands.[3] Due to its stability, it is often utilized as a precursor en route to the synthesis of other organoplatinum compound, such as hydrosilylation catalysts.[4] It is also used as a precursor for forming platinum layers for electronics.[5]
Synthesis
The first reported procedure was in 1909 by Pope and Peachey, where they treated chloroplatinic acid with excess methylmagnesium iodide.[1] The trimethylated Pt(IV) complex could be isolated as a yellow crystalline solid, which contained iodine impurities.[6] Improvements on the synthesis in later years utilized the more facile hexachloroplatinate reagent, potassium hexachloroplatinate, and the addition of iodomethane to reduce the amount of methylmagnesium that needed to use, which generated more byproducts.[7]
Trimethylplatinum iodide could also be formed from ion exchange using potassium iodide, starting from other trimethylplatinum(IV) complexes such as (CH3)3Pt(NO3) or (CH3)3Pt(SO4).[6]
Structure
Crystallography
The compound exists as a tetramer, crystallizing in a monoclinic unit cell.[3] Each Pt atom is coordinated in pseudo-octahedral geometry, while the tetramer unit is nearly cubic in the Pt-I core. The average Pt-I bond distance is 2.83 Å, while the average Pt-C bond distance is 2.04 Å. The fluoride, bromide, chloride, and pseudohalide (OH, N3, SCN, SMe) analogues [(CH3)3PtX)]4 also exist as tetramers, all forming cubane clusters.[8]
Electronic
The tetramer absorbs in weakly at 436 nm and strongly in the UV (209 nm) region and exhibits a very weak emission of 735 nm at room temperature in the solid state or in glasses of toluene.[9] On the basis of octahedral symmetry, the lower energy absorption is suggested to be a spin-forbidden d-d transition, while the higher energy absorption is proposed to be a ligand-to-metal charge transfer band due to high oxidation state on the Pt. The emission is suggested to be phosphorescence, with metal-metal interactions leading to a large Stokes shift.
Infrared and Raman spectroscopy have also been used to indicate the tetramer structure with cubic breathing modes at very low energies of < 250 cm−1.[10]
Reactivity
The tetramer decomposes, sometime explosively, when heated below at 175 - 200°C, resulting in platinum metal, ethane, and iodomethane.[1] When exposed to UV light in solution, the tetramer can undergo photolysis of two methyl radicals by reductive elimination, forming the brief Pt(II)(CH3)I species.[9] This reactivity is attributed to the ligand-to-metal charge transfer excitation.
The cubane structure can undergo decomposition by ligand substitution and breaking of the bridging iodine, to form a variety of octahedral organoplatinum complexes. Derived compounds include those with polypyridine,[11] pincer,[12] acetylacetonate,[13] and trispyrazolylborate ligands.[14]
Other applications
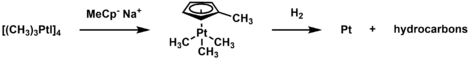
Trimethylplatinum iodide has been utilized as a precursor to plate Pt films through chemical vapor deposition or atomic layer deposition.[5] To do so, the complex is thermally decomposed, and the iodide is exchanged with methylcyclopentadienyl. The resulting complex (MeCp)Pt(CH3)3 sublimes near room temperature and, when reacted with hydrogen gas, can plate platinum metal. The plating can also be done in oxidative conditions.[15]
Like other platinum complexes, the trimethylplatinum iodide complex can lead to hydrosilylation of alkenes.[4] While the tetramer itself cannot catalyze hydrosilylation, when it is heated in dodecane in the presence of olefins and hydrosilanes, the subsequent decomposition of the tetramer can generate platinum species that can perform catalytic homogeneous hydrosilyation. The decomposition can be done photolytically in the crosslinking of silicon rubber.[16]
See also
References
- ↑ 1.0 1.1 1.2 Pope, William Jackson; Peachey, Stanley John (1909). "LXXIII.—The alkyl compounds of platinum" (in en). J. Chem. Soc., Trans. 95: 571–576. doi:10.1039/CT9099500571. ISSN 0368-1645. http://xlink.rsc.org/?DOI=CT9099500571.
- ↑ Clegg, D. E.; Hall, J. R.; Brubaker, C. H.; Gilbert, G. L.; Dyke, M. (2007-01-05), Muetterties, Earl L., ed., "Iodo(trimethyl)platinum(IV)", Inorganic Syntheses (Hoboken, NJ, USA: John Wiley & Sons, Inc.): pp. 71–74, doi:10.1002/9780470132418.ch13, ISBN 978-0-470-13241-8, https://onlinelibrary.wiley.com/doi/10.1002/9780470132418.ch13, retrieved 2023-03-11
- ↑ 3.0 3.1 Donnay, G.; Coleman, L. B.; Krieghoff, N. G.; Cowan, D. O. (1968-01-23). "Trimethylplatinum(IV) iodide and its misrepresentation as hexamethyldiplatinum". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 24 (1): 157–159. doi:10.1107/S056774086800186X. https://scripts.iucr.org/cgi-bin/paper?S056774086800186X.
- ↑ 4.0 4.1 Pratt, Sandra L.; Faltynek, Roberta A. (1984). "Studies on the thermal activation of iodotrimethylplatinum(IV) tetramer: hydrosilation catalyst generation from an inert precursor" (in en). Journal of Molecular Catalysis 24 (1): 47–58. doi:10.1016/0304-5102(84)85038-5. https://linkinghub.elsevier.com/retrieve/pii/0304510284850385.
- ↑ 5.0 5.1 Xue, Ziling; Strouse, M. Jane; Shuh, David K.; Knobler, Carolyn B.; Kaesz, Herbert D.; Hicks, Robert F.; Williams, R. Stanley (1989). "Characterization of (methylcyclopentadienyl)trimethylplatinum and low-temperature organometallic chemical vapor deposition of platinum metal" (in en). Journal of the American Chemical Society 111 (24): 8779–8784. doi:10.1021/ja00206a002. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00206a002.
- ↑ 6.0 6.1 Hoff, G. R.; Brubaker, C. H. (1968). "Some properties of several trimethylplatinum(IV) compounds" (in en). Inorganic Chemistry 7 (8): 1655–1656. doi:10.1021/ic50066a041. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic50066a041.
- ↑ , Angelino; Ralf Karch & Eileen Woerner"Trimethylplatinum(iv) iodide" patent WO2021186087A1, issued 2021-09-23
- ↑ Donath, H; Avtomonov, E. V; Sarraje, I; von Dahlen, K. -H; El-Essawi, M; Lorberth, J; Seo, B. -S (1998-05-29). "Organoplatinum compounds VII1For part VI see Ref. ([4b).1: Trimethylplatinum fluoride [(CH3)3PtF]4, the missing link in organoplatinum cluster chemistry: its synthesis, crystal structure and a comparison to the crystal structure of [(CH3)3PtOH]4"] (in en). Journal of Organometallic Chemistry 559 (1): 191–196. doi:10.1016/S0022-328X(98)00481-1. ISSN 0022-328X. https://www.sciencedirect.com/science/article/pii/S0022328X98004811.
- ↑ 9.0 9.1 Kunkely, H.; Vogler, A. (1991). "Electronic spectra and photochemistry of methyl platinum(IV) complexes" (in en). Coordination Chemistry Reviews 111: 15–25. doi:10.1016/0010-8545(91)84006-Q. https://linkinghub.elsevier.com/retrieve/pii/001085459184006Q.
- ↑ Clegg, D. E.; Hall, J. R. (1970-04-01). "Vibrational spectra of chloro-, bromo- and iodotrimethylplatinum(IV)" (in en). Journal of Organometallic Chemistry 22 (2): 491–496. doi:10.1016/S0022-328X(00)86069-6. ISSN 0022-328X. https://www.sciencedirect.com/science/article/pii/S0022328X00860696.
- ↑ Ghosh, Biswa Nath; Topić, Filip; Sahoo, Prasit Kumar; Mal, Prasenjit; Linnera, Jarno; Kalenius, Elina; Tuononen, Heikki M.; Rissanen, Kari (2014-12-02). "Synthesis, structure and photophysical properties of a highly luminescent terpyridine-diphenylacetylene hybrid fluorophore and its metal complexes" (in en). Dalton Transactions 44 (1): 254–267. doi:10.1039/C4DT02728K. ISSN 1477-9234. PMID 25373423. https://pubs.rsc.org/en/content/articlelanding/2015/dt/c4dt02728k.
- ↑ Mazzeo, Mina; Strianese, Maria; Kühl, Olaf; Peters, Jonas C. (2011-08-23). "Phosphido pincer complexes of platinum: synthesis, structure and reactivity" (in en). Dalton Transactions 40 (35): 9026–9033. doi:10.1039/C1DT10825E. ISSN 1477-9234. PMID 21826355. https://pubs.rsc.org/en/content/articlelanding/2011/dt/c1dt10825e.
- ↑ Zharkova, G. I.; Baidina, I. A.; Naumov, D. Yu.; Igumenov, I. K. (2011-06-01). "Crystal structure of volatile pyridine adducts of trimethylplatinum(IV) β-diketonates" (in en). Journal of Structural Chemistry 52 (3): 550–555. doi:10.1134/S0022476611030152. ISSN 1573-8779. https://doi.org/10.1134/S0022476611030152.
- ↑ Kuchta, Matthew C.; Gemel, Christian; Metzler-Nolte, Nils (2007-02-15). "An amino acid bioconjugate of an organoplatinum tris(pyrazolyl)borate complex: Synthesis and structure of [p-(tBuO–Phe–CO)C6H4TpPtMe3"] (in en). Journal of Organometallic Chemistry. Third International Symposium on Bioorganometallic Chemistry 692 (6): 1310–1314. doi:10.1016/j.jorganchem.2006.10.028. ISSN 0022-328X. https://www.sciencedirect.com/science/article/pii/S0022328X06008655.
- ↑ Hiratani, Masahiko; Nabatame, Toshihide; Matsui, Yuichi; Imagawa, Kazushige; Kimura, Shinichiro (2001). "Platinum Film Growth by Chemical Vapor Deposition Based on Autocatalytic Oxidative Decomposition" (in en). Journal of the Electrochemical Society 148 (8): C524. doi:10.1149/1.1381389. Bibcode: 2001JElS..148C.524H. https://iopscience.iop.org/article/10.1149/1.1381389.
- ↑ , Wolfgang & Andreas Köllnberger"Platinum Complexes and Their Use in Compounds That Can Be Cross-Linked by a Hydrosilylation Reaction" patent, issued 2016-03-03
 |




