Chemistry:Triphenylphosphine phenylimide
From HandWiki
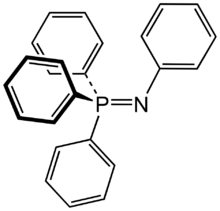
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetraphenylphosphanimine | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H20NP | |
| Molar mass | 353.405 g·mol−1 |
| Appearance | White solid |
| Density | 1.239 g/cm3 |
| Melting point | 131–132 °C (268–270 °F; 404–405 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triphenylphosphine phenylimide is the organophosphorus compound with the formula Ph3P=NPh (Ph = C6H5). It is a white solid that is soluble in organic solvents. The compound is a prototype of a large class of Staudinger reagents, resulting from the Staudinger reaction.
The phosphine imides were first prepared in the laboratory of Nobelist Hermann Staudinger. His synthesis involved the direct reaction of triphenylphosphine with phenylazide. [1]
- Ph3P + N3Ph → Ph3P=NPh + N2
X-ray crystallography establishes that the P-N-C angle is bent (130.4°) and the P-N distance is 160 pm.[2]
References
- ↑ Staudinger, H; Meyer, Jules (1919). "Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine". Helvetica Chimica Acta 2: 635–646. doi:10.1002/hlca.19190020164. https://zenodo.org/record/1426801.
- ↑ Eberhard Böhm; Kurt Dehnicke; Johannes Beck; Wolfgang Hiller; Joachim Strähle; Andreas Maurer; Dieter Fenske (1988). "Die Kristallstrukturen von Ph3PNPh, [Ph3PN(H)Ph][AuI2], und von 2,3-Bis(triphenylphosphoranimino)maleinsäure-N-methylimid (The Crystal Structures of Ph3PNPh, [Ph3PN(H)Ph][AuI2] and of 2,3-Bis(triphenylphosphoranimino)maleic Acid-N-methylimide)". Zeitschrift für Naturforschung B 43 (2): 138–148. doi:10.1515/znb-1988-0202.
 |
