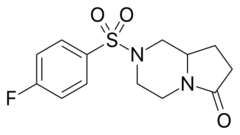
Chemistry:Unifiram
 | |
 | |
| Clinical data | |
|---|---|
| Other names | DM-232 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H15FN2O3S |
| Molar mass | 298.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
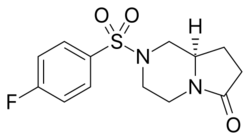
Unifiram (developmental code name DM-232) is an experimental drug.[1] that has antiamnesic and other effects in animal studies with far greater potency than piracetam.[2][3] A number of related compounds are known, such as sunifiram (DM-235) and sapunifiram (MN-19).[4][5][6] Unifiram has two enantiomers, with the dextro form being the more active isomer.[7] It has been shown to reduce the duration of hypnosis induced by pentobarbital, without impairing motor coordination.[8] As of 2015[update], no formal human studies with unifiram have been conducted. Unifiram is not patented and, despite the lack of human and long-term toxicity studies, it is commonly sold online.[9]
Pharmacology
Unifiram, as well as sunifiram, were assayed at a wide panel of sites, including the most important receptors, ion channels, and transporters, but showed no affinity for any of the sites.[9][3] They specifically did not bind to the glutamate, GABA, serotonin, dopamine, adrenergic, histamine, acetylcholine, or opioid receptors at concentrations of up to 1 μM.[9][3] In addition, the drugs were tested on recombinant AMPA receptors and showed no potentiation of the receptors, indicating that they do not act as AMPA receptor positive allosteric modulators.[9] However, they were able to prevent the amnesia induced by the AMPA receptor antagonist NBQX in the passive avoidance test, suggesting that indirect/downstream AMPA receptor activation may be involved in their memory-enhancing effects.[3]
Chemistry
References
- ↑ "AMPA-receptor activation is involved in the antiamnesic effect of DM 232 (unifiram) and DM 235 (sunifiram)". Naunyn-Schmiedeberg's Archives of Pharmacology 368 (6): 538–545. December 2003. doi:10.1007/s00210-003-0812-6. PMID 14600801.
- ↑ "DM235 (sunifiram): a novel nootropic with potential as a cognitive enhancer". Naunyn-Schmiedeberg's Archives of Pharmacology 365 (6): 419–426. June 2002. doi:10.1007/s00210-002-0577-3. PMID 12070754.
- ↑ 3.0 3.1 3.2 3.3 "Pharmacological characterization of DM232 (unifiram) and DM235 (sunifiram), new potent cognition enhancers". CNS Drug Reviews 12 (1): 39–52. 2006. doi:10.1111/j.1527-3458.2006.00039.x. PMID 16834757.
- ↑ "Structure-activity relationship studies on unifiram (DM232) and sunifiram (DM235), two novel and potent cognition enhancing drugs". Bioorganic & Medicinal Chemistry 12 (1): 71–85. January 2004. doi:10.1016/j.bmc.2003.10.025. PMID 14697772.
- ↑ "Design, synthesis and preliminary pharmacological evaluation of new piperidine and piperazine derivatives as cognition-enhancers". Bioorganic & Medicinal Chemistry 16 (3): 1431–1443. February 2008. doi:10.1016/j.bmc.2007.10.050. PMID 17981042.
- ↑ "Design, synthesis and preliminary pharmacological evaluation of new analogues of DM232 (unifiram) and DM235 (sunifiram) as cognition modulators". Bioorganic & Medicinal Chemistry 16 (23): 10034–10042. December 2008. doi:10.1016/j.bmc.2008.10.017. PMID 18954993.
- ↑ "Enantioselective synthesis and preliminary pharmacological evaluation of the enantiomers of unifiram (DM232), a potent cognition-enhancing agent". Medicinal Chemistry 1 (5): 473–480. September 2005. doi:10.2174/1573406054864142. PMID 16787332.
- ↑ "The novel nootropic compound DM232 (UNIFIRAM) ameliorates memory impairment in mice and rats". Drug Development Research 56: 23–32. 2002. doi:10.1002/ddr.10055.
- ↑ 9.0 9.1 9.2 9.3 "Unifi nootropics from the lab to the web: a story of academic (and industrial) shortcomings". Journal of Enzyme Inhibition and Medicinal Chemistry 31 (2): 187–194. 2016. doi:10.3109/14756366.2015.1021252. PMID 25831025. https://figshare.com/articles/journal_contribution/2065971.
 |
