Chemistry:Urushiol
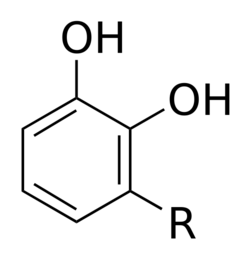 R = (CH2)14CH3, (CH2)7CH=CH(CH2)5CH3, (CH2)7CH=CHCH2CH=CH(CH2)2CH3, (CH2)7CH=CHCH2CH=CHCH=CHCH3, (CH2)7CH=CHCH2CH=CHCH2CH=CH2 and more. |
Urushiol /ʊˈruːʃi.ɒl/ is an oily mixture of organic compounds with allergenic properties found in plants of the family Anacardiaceae, especially Toxicodendron spp. (e.g., poison oak, Chinese lacquer tree, poison ivy, poison sumac), Comocladia spp. (maidenplums), Metopium spp. (poisonwood), and also in parts of the mango tree as well as the fruit of the cashew tree.[1][2][3][4][5][6]
In most individuals, urushiol causes an allergic skin rash on contact,[7] known as urushiol-induced contact dermatitis.
The name urushiol is derived from the Japanese word for the lacquer tree, Toxicodendron vernicifluum (漆 urushi).[8] The oxidation and polymerization of urushiol in the tree's sap in the presence of moisture allows it to form a hard lacquer, which is used to produce traditional Chinese, Korean, and Japanese lacquerware.
History
Although urushiol-containing lacquers and their skin-irritating properties were well known in East Asia for several millennia beforehand, its first recorded Western texts were in 1624 by John Smith where he initially likened poison ivy to English ivy.[9] He did not classify it as a poison at first due to the speed with which its rash disappeared and Smith hypothesized that there may actually be medicinal uses for the plant.[9] In the 18th and 19th centuries, many experiments were done in this area to determine whether or not this theory was true. Because medicine was dominated by plant-based treatments at the time, physicians were hopeful that the strong effect this chemical produced on the body could be effective in some way.
André-Ignace-Joseph Dufresnoy was one of the first to come up with a medicinal use for this chemical in 1780 when he boiled poison ivy to produce an infusion for internal use.[9] This led to a distilled extract of poison ivy which he prescribed to many people suffering from skin problems and even paralysis. He claimed this treatment to have yielded several positive results.[9]
For many years, poison ivy was thought to fall into the Rhus genus; however, in the 1900s, it was reclassified into a more appropriate genus, Toxicodendron, meaning poison tree.[9] There were many documented cases of irritations and allergic reactions from the plant, and its prevalence in medicinal use quickly dwindled. After this new categorization, scientists began attempts to determine what it was that rendered plants of this genus noxious, starting with a hypothesis of a volatile oil present in the plants.[9]
While this proved incorrect, Rikou Majima from Japan was able to determine that the chemical urushiol was the irritant. Further, he determined that the substance was a type of alkyl catechol, and due to its structure it was able to penetrate the skin and survive on surfaces for months to years.[9] Urushiol's ability to polymerise into a hard glossy coating is the chemical basis for traditional lacquerware in many Asian countries.[10] After urushiol comes in contact with oxygen, under certain conditions it becomes a black lacquer and has been named urushi lacquer.[11]
Characteristics
Urushiol in its pure form is a pale-yellow liquid with a density of about 0.968 g/mL and a boiling point of 200 °C (392 °F). It is soluble in diethyl ether, acetone, ethanol, carbon tetrachloride, and benzene.[12][13]
Urushiol is a mixture of several closely related organic compounds. Each consists of a catechol substituted in the 3 position with a hydrocarbon chain that has 15 or 17 carbon atoms. The hydrocarbon group may be saturated or unsaturated. The exact composition of the mixture varies, depending on the plant source. Whereas western poison oak urushiol contains chiefly catechols with C17 side-chains,[14] poison ivy and poison sumac contain mostly catechols with C15 sidechains.
The likelihood and severity of allergic reaction to urushiol is dependent on the degree of unsaturation of the hydrocarbon chain. Less than half of the general population experience a reaction with the saturated urushiol alone, but over 90% do so with urushiol that contains at least two degrees of unsaturation (double bonds). Longer side chains tend to produce a stronger reaction.[15]
Urushiol is an oleoresin contained within the sap of poison ivy and related plants, and after injury to the plant, or late in the fall, the sap leaks to the surface of the plant, where under certain temperature and humidity conditions the urushiol becomes a blackish lacquer after being in contact with oxygen.[15][16][11] Urushi lacquer is very stable. It is able to withstand disturbances from alkali, acid, and alcohol, while also being able to resist temperatures of over 300 °C. However, the lacquer can be degraded by UV rays from the sun and other sources.[10]
Within the United States, urushiol-containing plants are widely distributed. Poison ivy can be found in all states except California, Alaska, and Hawaii. Poison Oak can be found on the west coast or in some states in the southeast, while poison sumac can be found only in the eastern half of the country.[17]
These plants all have distinguishing features that will help in identification. Poison ivy always grows with groups of three glossy, pointed leaflets. Poison oak has a similar appearance, but with larger and more rounded lobes, with a light, velvety pubescence and grow in groups of 3, 5, or 7. Poison sumac grows in groups of 7 to 13 leaflets, always an odd number. The leaflets are ovate-lanceolate and glossy.[18]
Allergic response and treatment
Before the urushiol has been absorbed by the skin, it can be removed with soap and water. Substantial amounts of urushiol may be absorbed within minutes. Once urushiol has penetrated the skin, attempting to remove it with water is ineffective.[15] After being absorbed by the skin, it is recognized by the immune system's dendritic cells, otherwise called Langerhans cells. These cells then migrate to the lymph nodes, where they present the urushiol to T-lymphocytes and thus recruit them to the skin, and the T-lymphocytes cause pathology through the production of cytokines and cytotoxic damage to the skin.[19] This causes the painful rash, blisters, and itching.
Once this response starts, only a few treatments, such as cortisone or prednisone, are effective.[9] Medications that can reduce the irritation include antihistamines (diphenhydramine (Benadryl) or cetirizine (Zyrtec)). Other treatments include applying cold water or calamine lotion to soothe the pain and stop the itching.[9]
Mechanism of action
File:How Poison Ivy Works.webm
To cause an allergic dermatitis reaction, the urushiol is first oxidized to create two double-bonded oxygens on the chemical. It then reacts with a protein nucleophile to trigger a reaction within the skin. Dermatitis is mediated by an acquired immune response. Urushiol is too small a molecule to directly activate an immune response. Instead, it attaches to certain proteins of the skin, where it acts as a hapten, leading to a type IV hypersensitivity reaction.[20][self-published source?][21]
Hydrocortisone, the active ingredient in cortisone, works to alleviate this condition by stopping the release of chemicals that cause the dermatitis reaction.[22] Hydrocortisone itself does not react with urushiol in any way.

See also
- Antipruritics, for treating the toxin.
- Bentoquatam, a barrier cream applied before exposure.
- Burow's solution which can treat the symptoms of the rash caused by urushiol.
- Ginkgo biloba and cashew, plants containing chemicals closely related to urushiol.
- Hapten, small molecules that can elicit an immune response under certain conditions.
- Mango trees, which may cause cross-reaction allergies with urushiol.
References
- ↑ Cruse, Julius M.; Lewis, Robert E. (2003). Atlas of Immunology, Second Edition. CRC Press. p. 375. ISBN 978-1-4200-3994-8. https://books.google.com/books?id=L4CI-qkhuQ8C&pg=PA375.
- ↑ "Can Reaction to Poison Ivy Cause Mango Allergy?". American College of Allergy, Asthma and Immunology. http://www.acaai.org/allergist/Resources/ask-allergist/Pages/Poison_Ivy_Mango.aspx.
- ↑ "Urushiol: Human Health Effects". NIH. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+7485.
- ↑ Rietschel, Robert L.; Fowler, Joseph F.; Fisher, Alexander A. (2008). Fisher's Contact Dermatitis. PMPH-USA. p. 407. ISBN 978-1-55009-378-0. https://books.google.com/books?id=dQBAzfyCeQ8C&pg=PA407.
- ↑ Appleby, Maia (Aug 2013). "Mango & Skin Rashes". Livestrong. http://www.livestrong.com/article/179501-mango-skin-rashes/.
- ↑ Aguilar-Ortigoza, Carlos J.; Sosa, Victoria; Aguilar-Ortigoza, Marcial (2003-09-01). [0354:TPIVAS2.0.CO;2 "Toxic Phenols in various Anacardiaceae species"] (in en). Economic Botany 57 (3): 354. doi:10.1663/0013-0001(2003)057[0354:TPIVAS2.0.CO;2]. ISSN 1874-9364. https://doi.org/10.1663/0013-0001(2003)057[0354:TPIVAS]2.0.CO;2.
- ↑ Tilton, Buck (2004). Wilderness First Responder: How to Recognize, Treat, and Prevent Emergencies in the Backcountry. Globe Pequot. ISBN 978-0-7627-2801-5.
- ↑ Oxford English Dictionary[full citation needed]
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 "No Ill Nature: The Surprising History and Science of Poison Ivy and Its Relatives" (in en). 2013-06-02. https://www.sciencehistory.org/distillations/no-ill-nature-the-surprising-history-and-science-of-poison-ivy-and-its-relatives.
- ↑ 10.0 10.1 Arney, Kat (13 June 2017). "Urushiol". Education in Chemistry (Royal Society of Chemistry) 54 (4): 8. https://eic.rsc.org/magnificent-molecules/urushiol/3007556.article. Retrieved 19 June 2018.
- ↑ 11.0 11.1 Barceloux, Donald G. (2008). Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals. John Wiley and Sons. pp. 681–. ISBN 978-0-471-72761-3. https://books.google.com/books?id=CpqzhHc072AC&pg=PA681. Retrieved 2010-07-26.
- ↑ Hawley's Condensed Chemical Dictionary (14th ed.). John Wiley & Sons. 2002. http://www.knovel.com/web/portal/knovel_content?p_p_id=EXT_KNOVEL_CONTENT&p_p_action=1&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_EXT_KNOVEL_CONTENT_struts_action=/ext/knovel_content/view&_EXT_KNOVEL_CONTENT_contentType=2&_EXT_KNOVEL_CONTENT_SpaceID=0&_EXT_KNOVEL_CONTENT_VerticalID=0&_EXT_KNOVEL_CONTENT_SetID=5316942&_EXT_KNOVEL_CONTENT_BookID=704&_EXT_KNOVEL_CONTENT_NodeID=1884538029&_EXT_KNOVEL_CONTENT_Associated=true&sistring=&ststring=.
- ↑ Howell, J. B. (1 October 1941). "Solubility of the Dermatitis-Producing Fraction of Poison Ivy". Archives of Dermatology 44 (4): 665. doi:10.1001/archderm.1941.01500040120010.
- ↑ Hogan, C. Michael (2008). "Western poison-oak: Toxicodendron diversilobum". in Stromberg, Nicklas. http://globaltwitcher.auderis.se/artspec_information.asp?thingid=82914.
- ↑ 15.0 15.1 15.2 McGovern, Thomas; Barkley, Theodore (1998). "Botanical Dermatology". International Journal of Dermatology 37 (5): 321–334. doi:10.1046/j.1365-4362.1998.00385.x. PMID 9620476.
- ↑ Rietschel, Robert L.; Fowler, Joseph F.; Fisher, Alexander A. (2008). Fisher's Contact Dermatitis. PMPH-USA. p. 408. ISBN 978-1-55009-378-0. https://books.google.com/books?id=dQBAzfyCeQ8C&pg=PA408. Retrieved 2010-07-26.
- ↑ "Poisonous Plants – Geographic Distribution | NIOSH | CDC" (in en-us). 2020-02-21. https://www.cdc.gov/niosh/topics/plants/geographic.html.
- ↑ "Slideshow: Images of Poison Ivy, Poison Oak, Poison Sumac" (in en). https://www.webmd.com/allergies/ss/slideshow-poison-plants.
- ↑ Gober, D. Michael (2008). "Human Natural Killer T Cells infiltrate into the Skin at Elicitation Sites of Allergic Contact Dermatitis". Journal of Investigative Dermatology 128 (6): 1460–1469. doi:10.1038/sj.jid.5701199. PMID 18079745.
- ↑ "Forget 'Polytetrafluoroethene', Pentadecacatechol is where it's at". 2012-07-11. http://journalclubscienceblog.wordpress.com/2012/07/11/forget-polytetrafluoroethene-pentadecacatechol-is-where-its-at-3-2/.
- ↑ Marwa, K; Kondamudi, NP (2021-01-01). "Type IV Hypersensitivity Reaction". StatPearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK562228/#_NBK562228_pubdet_. Retrieved 2021-11-28.
- ↑ "Hydrocortisone cream: a steroid medicine" (in en). 2019-01-17. https://www.nhs.uk/medicines/hydrocortisone-skin-cream/.
Further reading
- Grevelink, Suzanne A.; Murrell, Dédée F.; Olsen, Elise A. (August 1992). "Effectiveness of various barrier preparations in preventing and/or ameliorating experimentally produced Toxicodendron dermatitis". Journal of the American Academy of Dermatology 27 (2): 182–188. doi:10.1016/0190-9622(92)70167-e. PMID 1430354.
- Symes, William F.; Dawson, Charles R. (June 1954). "Poison Ivy 'Urushiol'". Journal of the American Chemical Society 76 (11): 2959–2963. doi:10.1021/ja01640a030.
- Xu, Hongli; Lu, Zaijun; Zhang, Guangzhao (2012). "Synthesis and properties of thermosetting resin based on urushiol". RSC Advances 2 (7): 2768. doi:10.1039/C2RA00829G. Bibcode: 2012RSCAd...2.2768X.
- Kalish, RS; Johnson, KL (1 December 1990). "Enrichment and function of urushiol (poison ivy)-specific T lymphocytes in lesions of allergic contact dermatitis to urushiol". Journal of Immunology 145 (11): 3706–3713. doi:10.4049/jimmunol.145.11.3706. INIST:4566503 NAID 80005581100. PMID 2147199.
- Xia, Jianrong; Xu, Yanlian; Lin, Jinhuo; Hu, Binghuan (January 2008). "UV-induced polymerization of urushiol without photoinitiator". Progress in Organic Coatings 61 (1): 7–10. doi:10.1016/j.porgcoat.2007.08.007.
- Epstein, William L. (1 March 1974). "Poison Oak Hyposensitization: Evaluation of Purified Urushiol". Archives of Dermatology 109 (3): 356–360. doi:10.1001/archderm.1974.01630030016002. PMID 4273614.
- Je, Hyeondoo; Won, Jongok (January 2021). "Natural urushiol as a novel under-water adhesive". Chemical Engineering Journal 404: 126424. doi:10.1016/j.cej.2020.126424.
- Oshima, Ryuichi; Yamauchi, Yoshio; Watanabe, Chuichi; Kumanotani, Ju (July 1985). "Enzymic oxidative coupling of urushiol in sap of the lac tree, Rhus vernicifera". The Journal of Organic Chemistry 50 (15): 2613–2621. doi:10.1021/jo00215a002.
External links
- Poison Oak at Wayne's Word
- Reduce poison ivy dermatitis with the following steps – Deseret News
- The Poison Ivy Tutorial
 |
